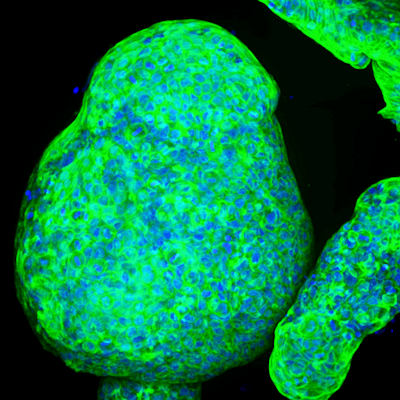
September 22, 2022 -- A team of scientists combined organoids with organ-on-a-chip technology to replicate the disease process underlying autosomal recessive polycystic kidney disease (APRKD). The new modeling system identified two potential therapeutics for APRKD, which currently has no U.S. Food and Drug Administration (FDA)-approved treatment.
The group from Massachusetts General Hospital, Brigham and Women's Hospital, and the Wyss Institute used a 3D printer to create a perfusion chip that models the microenvironment of the cells within the kidney and allows liquid to flow through the organoids (Science Advances, September 21, 2022).
Previous attempts to model APRKD were thwarted because while scientists could cultivate mutated PKHD1 cells responsible for the disease, they weren't able to replicate urinary flow. However, by uniting an organoid with a perfusion chip, the researchers were able to recreate the microenvironment present in humans. They found that two mechanosensing molecules (FOS and RAC1) are potential therapeutic targets for APRKD.
The study revealed the molecule FOS may be a crucial determent of species-specific cyst formation, which explains why mouse models were unable to replicate the disease effectively. The study also demonstrated why mutations in the PKHD1 gene lead to cyst formation. The scientists used FDA-approved drugs R-Naproxen and R-Ketorolact to inhibit RAC1 and one investigational new drug that inhibits FOS (T-5224).
All three drugs were shown to have therapeutic effects and the study highlights the potential of organoid-on-a-chip models to elucidate other complex disease mechanisms for therapeutic testing and discovery, according to the researchers.
Copyright © 2022 scienceboard.net