May 16, 2023 -- Researchers at NIH’s National Institute of Allergy and Infectious Diseases (NIAIDs) have developed an experimental universal influenza vaccine.
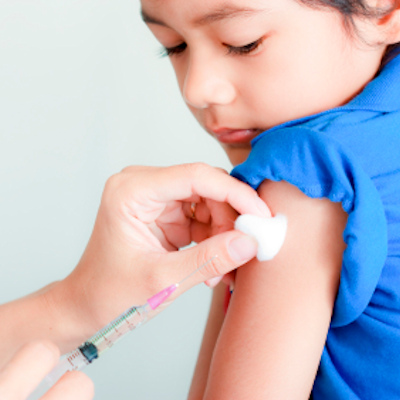
They are enrolling volunteers for a phase I clinical trial to test the vaccine, called H1ssF-3928 mRNA-LNP, for its safety and efficacy.
The Centers for Disease Control and Prevention (CDC) estimates that between 12,000 and 52,000 U.S. citizens die of seasonal influenza, or flu, each year. Although annual seasonal flu vaccines help control the spread and severity of influenza, they do not provide immunity against every flu strain.
Each year, scientific experts must predict which influenza strains are likely to be most common in the upcoming flu season, and then select three or four to include in the next seasonal flu vaccine. During the time needed to produce this vaccine, dominant virus strains can morph unexpectedly, potentially decreasing the vaccine's efficacy.
The researchers sought to develop a universal flu vaccine that could eliminate these problems by protecting against a wide variety of strains and providing durable long-term immunity, thus eliminating the need for annual vaccines.
The vaccine uses a specific portion of a flu protein, hemagglutinin, to induce a broad immune response against influenza. While the head portion of the hemagglutinin protein tends to change as the flu virus spreads and evolves, a more stable portion, known as the stem, evolves very slowly and is very similar across many different types of the flu virus. By using the hemagglutinin stem as the basis for this vaccine, researchers hope to induce long-term immunity against a broad range of flu viruses.
Unlike earlier vaccines, the H1ssF-3928 mRNA-LNP vaccine candidate uses a messenger RNA (mRNA) platform. The researchers hypothesize that developing a variety of different platforms for a universal flu vaccine may increase their chances of finding one that provides safe, strong, broad immunity against a variety of strains.
The trial, occurring at Duke University, is enrolling up to 50 healthy volunteers, aged 18 through 49. Three groups of 10 participants each will be vaccinated with 10, 25, and 50 micrograms of the experimental vaccine, respectively. After evaluation to determine an optimum dosage, an additional 10 participants will receive the optimum dosage.
An additional participant group will receive a current seasonal influenza vaccine, allowing the researchers to directly compare the candidate vaccine with an available flu vaccine in terms of their safety and immunogenicity -- i.e., their ability to provoke an immune response in the body. Participants will be evaluated regularly to assess the new vaccine and will receive follow-up appointments for up to one year.
"A universal influenza vaccine would be a major public health achievement and could eliminate the need for both annual development of seasonal influenza vaccines, as well as the need for patients to get a flu shot each year," NIAID's acting director Dr. Hugh Auchincloss said in a statement. "A universal flu vaccine could also serve as an important line of defense against the spread of a future flu pandemic."
Copyright © 2023 scienceboard.net