July 1, 2022 -- A study funded by the National Institutes of Health (NIH) has shown that a new universal flu vaccine offers broad protection against different strains and subtypes of influenza A virus infections.
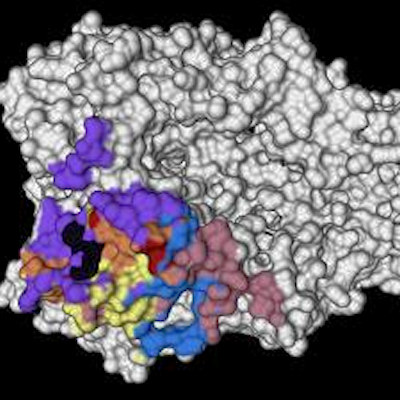
Researchers from the Institute for Biomedical Sciences at Georgia State University (GSU) developed the universal flu vaccine by genetically linking two highly conserved portions of the virus -- the extracellular domain of matrix 2 (M2e) and the stalk protein found in influenza A H3N2 viruses.
Their published findings in the journal npj Vaccines showed that M2e-stalk protein vaccination induced broad protection against different influenza virus strains and subtypes by universal vaccine-mediated immunity in adult and aged mice.
"The M2e-stalk protein, for the first time, could be easily produced in bacterial cell cultures at high yields and was found to confer protection against heterologous and heterosubtypic cross-group subtype viruses (H1N1, H5N1, H9N2, H3N2, and H7N9) at similar levels in adult and aged mice," said Sang-Moo Kang, PhD, senior author of the study and a professor at the Institute for Biomedical Sciences at GSU, in a written statement.
Kang said the study's results "provide evidence that M2e-stalk genetic fusion proteins can be produced in a large scale at low cost and developed as a universal influenza A virus vaccine candidate for young and aged populations."
In separate but related news this week, NIH announced that a phase I clinical trial of a novel influenza vaccine has begun inoculating healthy adult volunteers at the agency's Health Clinical Center in Bethesda, Maryland. The single-site, placebo-controlled trial will test the safety of a candidate vaccine, developed by researchers at the National Institute of Allergy and Infectious Diseases (NIAID), and its ability to prompt immune responses in up to 100 people between the ages of 18 and 55.
The candidate vaccine, BPL-1357, is a whole-virus vaccine made up of four strains of non-infectious, chemically inactivated, low-pathogenicity avian flu virus. An animal study, led by NIAID investigator Dr. Jeffery Taubenberger, PhD, and posted online as a preprint, found that all mice receiving two doses of BPL-1357 vaccine delivered either intramuscularly or intranasally survived later exposure to lethal doses of each of six different influenza virus strains, including subtypes that were not included in the vaccine.
NIH reports that similar results were obtained in challenge experiments in which ferrets were vaccinated with BPL-1357.
Copyright © 2022 scienceboard.net