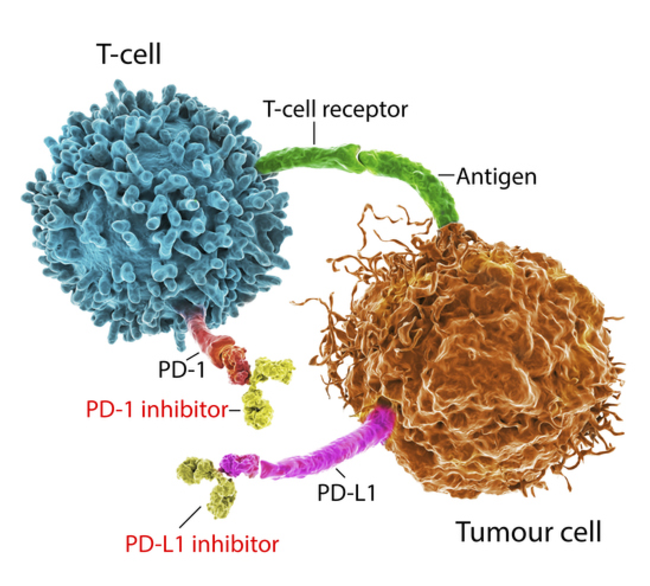
January 11, 2023 -- Southern China researchers unexpectedly found pre-surgery immunotherapy for a common type of colorectal cancer even more effective at early and mid-stages than its previously known high success rate in the metastatic stage of the disease. The study, published January 11 in JNCCN - Journal of the National Comprehensive Cancer Network, found that immune checkpoint programmed cell death 1 (PD-1) inhibitor treatment prior to surgery was significantly effective for patients with certain types of colorectal cancer.
The study included a retrospective review of 73 patients between the ages of 18 and 75, all of whom had confirmed mismatch repair-deficient/microsatellite instability-high (dMMR/MSI-H) colorectal cancer.
Mismatch repair-deficient (dMMR) tumor cells have an inability to correct DNA replication errors, resulting in the accumulation of mutation loads and the generation of neoantigens, which stimulate the host's antitumor immune response. Microsatellite instability-high (MSI-H) cancer cells have high numbers of mutations within tracts of repetitive DNA called microsatellites, indicating significant instability within the tumor. While dMMR/MSI-H represents a good prognosis in early colorectal cancer settings without adjuvant treatment, it generally represents a poor prognosis in patients with metastasis.
Of the 73 patients in the study, 48 were diagnosed with colon cancer, 18 with rectal cancer, and 7 with multiple colorectal cancers. All had received some type of PD-1 inhibitor prior to surgery between 2017 and 2021. An overall 84.9% experienced positive responses, with 23.3% showing complete response and 61.6% partial response. The two-year rates for tumor-specific overall survival and disease-free survival were 100% for patients who underwent surgery after PD-1 treatment.
The study's average follow-up time was 17.2 months, with 16 patients tracked for more than two years. Nearly all patients benefited from neoadjuvant PD-1 inhibitors, with 25% experiencing complete responses. In addition to the short-term effectiveness, the findings showed substantial longer survival benefits from neoadjuvant PD-1 inhibitors, including low recurrence compared with historic rates.
The researchers had anticipated PD-1 inhibitors could be at least as effective for locally-advanced but operable cancer as they have historically been in treating metastatic MSI-H colorectal cancer, but did not expect to find it as effective as it was for this patient population. They call for additional studies with longer follow-up periods to confirm these results, as well as to confirm this approach's long-term safety and its possible implications for limiting or avoiding surgery.
"I think care providers, especially surgeons, should refrain from scheduling immediate surgery for patients with locally advanced, or even early-stage dMMR/MSI-H colorectal cancer," said Sun Yat-sen University Cancer Center's senior author Dr. Pei-Rong Ding in a statement. "With such a powerful option at hand, we have the duty to offer a safer surgery with better outcomes or a nonsurgical yet equally effective approach for this group of patients, especially for those who might suffer from function damage or organ sacrifice after surgery. We need to keep in mind that our final goal is to cure patients long term, not just remove the tumor at the moment."
Copyright © 2023 scienceboard.net