January 29, 2020 -- Scientists have engineered a nanoparticle that eats plaques associated with cardiovascular disease -- from the inside out. This Trojan horse technology could offer a new therapeutic option for the treatment of atherosclerosis, according to a study published in Nature Nanotechnology on January 27.
Cardiovascular disease is one of the deadliest diseases in the world. Currently available therapies only target risk factors such as hypertension and hyperlipidemia instead of inhibiting the disease-causing pathways associated with atherosclerosis.
Atherosclerosis is caused by the buildup of arterial plaque, with plaques characterized by accumulation of apoptotic cells in a necrotic core. The body's natural process of removing dead cells by phagocytosis -- efferocytosis -- is defective in atherosclerosis.
Therefore, researchers are exploring the use of prophagocytic antibody-based therapies to stimulate the clearance of apoptotic cells. One challenge of using these therapies is that they can miss apoptotic cells and target healthy tissue, potentially leading to toxicities such as anemia.
Seeking to overcome this phenomenon, researchers from Michigan State University and Stanford University engineered a Trojan horse system of nanoparticles that accumulate in plaque phagocytes and reactivate efferocytosis locally to reduce plaque burden in arteries.
The system is composed of a macrophage-specific nanotherapy based on single-walled carbon nanotubes loaded with a chemical inhibitor of the antiphagocytic CD47-signal regulatory protein alpha (SIRPα) signaling axis. The CD47/SIRPα axis is a critical immune checkpoint involved in the development of certain white blood cells, specifically monocytes and macrophages. Monocytes are the primary circulating cells recruited to the diseased artery, where they differentiate into lesional macrophages, which become impaired in atherosclerosis.
SIRPα activates Src homology 2 domain-containing phosphatase-1 (SHP1) to mediate signaling that suppresses phagocytic function, ultimately leading to plaque formation. So, the researchers selected SHP1 inhibitor (SHP1i) as the prodrug in their system.
In the current study, the nanoparticle system works from the inside out. The nanoparticles were taken up by macrophages in lesions, and the SHP1i inhibited CD47-induced signaling by stimulating the clearance of diseased vascular cells. Specifically, the system significantly decreased the phosphorylation of SHP1, allowing for restored phagocytic function. The researchers also found that mice treated with the system had reduced vascular inflammation, which is associated with lower risk of developing cardiovascular disease.
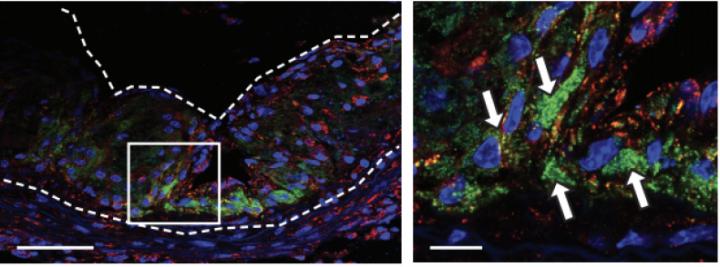
Remarkably, the nanoparticles are selective, with uptake by over 95% of macrophages compared with other cells. This offers an elegant solution to the negative off-target effects of other antibody-based therapies. Moreover, the researchers demonstrated that the nanoparticles accumulated in diseased cells, with minimal accumulation in other organs. The system was also validated as nontoxic, safely eliminated by immune cell peroxidases in a number of weeks.
The researchers used large-scale single-cell RNA sequencing to determine which inflammatory genes in lesional macrophages contributed to the pro-efferocytotic effect. They found that upstream regulators SIRPA and the SHP1 encoding gene, PTPN6, resulted in decreased pro-inflammatory transcripts and upregulation of genes linked to inflammatory resolution. Lesional macrophages were enriched with phagocytosis and antigen presentation genes.
The researchers hope to apply their nanoparticles to bispecific nanoimmunotherapies to apply multiple therapeutic agents. They have filed a provisional patent and will begin marketing it later this year.
"We were able to marry a groundbreaking finding in atherosclerosis by our collaborators with the state-of-the-art selectivity and delivery capabilities of our advanced nanomaterial platform. We demonstrated the nanomaterials were able to selectively seek out and deliver a message to the very cells needed," said co-author Bryan Smith, PhD, associate professor of biomedical engineering at Michigan State University in a statement. "It gives a particular energy to our future work, which will include clinical translation of these nanomaterials using large animal models and human tissue tests. We believe it is better than previous methods."
Do you have a unique perspective on your research related to disease research or bioengineering? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net






