April 26, 2021 -- A team of researchers has developed nanoparticles that use a completely different mode of action from conventional antibiotics. These inorganic nanoparticles have inherent antimicrobial properties and can target pesky bacteria that hide inside of cells. The results were published in Nanoscale on April 22.
An increasing number of bacterial strains are able to evade antibiotic treatment by hiding inside cells, where they can avoid detection and subsequent elimination by the host immune system. This strategy is known to be used by Staphylococci, which commonly infect human tissues, especially skin, bone, and other organs.
While these bacteria are mostly harmless, under certain conditions they can disseminate into the bloodstream and cause severe inflammation, and even lead to toxic shock or sepsis. This makes Staphylococci one of the main causes of death from single-pathogen infections. An increasing number of staphylococcal infections, particularly methicillin-resistant Staphylococcus aureus (MRSA), no longer respond to treatment with conventional antibiotics, resulting in high fatality rates.
One of the main limitations to treating MRSA infections may be the lack of access of antibiotics to the intracellular niche. Conventional antimicrobial agents are unable to penetrate infected mammalian cells and eliminate these bacteria. Moreover, while nanoparticles have shown promise for intracellular delivery of antibiotics (such as silver-based nanoparticles), these load-carrier systems are also limited by release prior to reaching the target site and systemic toxicity concerns.
Two-in-one effect
Inorganic nanoparticles with direct antibiotic activity have the potential to unite load and carrier functions of antimicrobial agents. In the past, silver-based systems have shown high antibiotic activity but can be toxic to mammalian cells.
But these inorganic systems may deserve a closer look. That's in part because the mostly unspecific nature of mechanisms of action (e.g., induction of oxidative stress, binding to proteins, and interruption of electron transport processes) makes bacteria less likely to develop resistance against the nanoparticles compared to conventional antibiotics, according to a team of researchers from the Swiss Federal Laboratories for Materials Science and Technology (EMPA) and the Swiss Federal Institute of Technology in Zurich (ETH Zurich), led by Inge Herrmann, PhD, and Tino Matter, PhD.
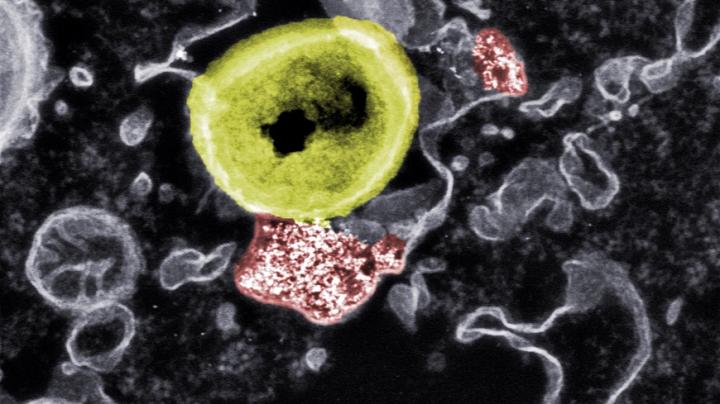
The researchers were interested in the use of bioglass, a bioactive ceramic material with versatile regenerative properties that is used for the reconstruction of bones and soft tissues. Bioglasses, specifically cerium oxide nanoparticles, have also demonstrated strong antimicrobial properties attributed to reversible conversion between its two valence states.
The chemical element cerium was named after the dwarf planet Ceres. The silvery metal, as cerium oxide, is commonly incorporated into car catalytic converters, and it is also used in the manufacture of products as diverse as self-cleaning ovens, windscreens, and LEDs.
The researchers developed inorganic hybrid metal oxide nanoparticles, based on cerium oxide, that unify direct antimicrobial activity with low mammalian cell toxicity. They produced a variety of metal-oxide nanoparticles based on bioglass (BG) alone, cerium alone, hybrids of the bioglass and cerium oxide (BG/ceria), and 2% zinc-doped strontium oxide (Sr)-substituted hybrids (Zn2-SrBG/ceria). Each was made in a single step using liquid-feed flame spray pyrolysis (LF-FSP), a highly scalable nanoparticle production method.
Scanning electron microscopy revealed that the nanoparticles were easily taken up into the same cellular components as bacteria due to their similar surface charge and size of bacteria. This allows the nanoparticles to unleash their activity in close proximity to the bacteria, according to the researchers.
Evidence that bioglass nanoparticles are effective against MRSA
Next, the team measured mitochondrial adenosine triphosphate (ATP) and released lactate dehydrogenase (LDH, an indicator for membrane damage) to determine cell viability and toxicity of the various nanoparticles, compared to the conventional small-molecule antibiotic, gentamicin. Whereas cell viability was greatly compromised with application of silver nanoparticles, the bioglass-based nanoparticles were well tolerated in cells, pointing to their high compatibility with human macrophages. The ceria bioglass nanoparticles also reduced intracellular MRSA counts where silver-based nanoparticles had no effect.
"What's more, the cerium particles regenerate over time, so that the oxidative effect of the nano-particles on the bacteria can start all over again," Matter said. In this way, the cerium particles could have a long-lasting effect, he explained.
To confirm that the antimicrobial effect of the nanoparticles is due to the internalization of the nanoparticles and subsequent intracellular colocalization with the bacteria, the researchers used high-angle annular dark-field (HAADF) scanning transmission electron microscopy. They confirmed that a direct interaction between intracellular nanoparticles and MRSA is required to eradicate the intracellular bacteria.
Importantly, during the interaction of nanoparticles and bacteria, mammalian cell membranes remained largely unaffected by the nanoparticles. This illustrates that the nanoparticles selectively disrupt bacterial membranes within the same cellular compartment.
In future work, the researchers would like to analyze the interactions of the nanoparticles in the infection process in more detail to further optimize the structure and composition of the nanoparticles and gain a more in-depth understanding of the mechanisms at play. The goal is to develop a simple, robust antibacterial agent that is effective inside infected cells.
Do you have a unique perspective on your research related to drug discovery and development? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net