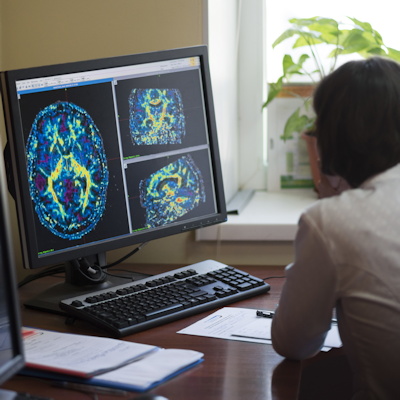
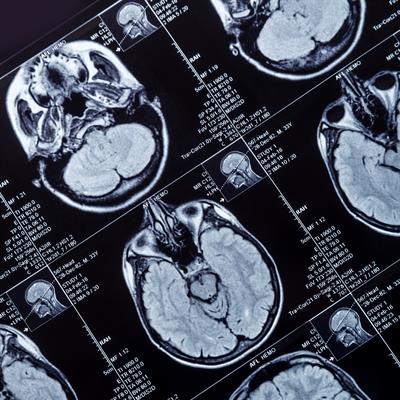
May 3, 2023 -- Northwestern Medicine scientists have used a novel, skull-implantable ultrasound device to allow the delivery of intravenously injected chemotherapy into the brain. The study, published Tuesday in the Lancet Oncology, represents the first human clinical trial to temporarily open the blood-brain barrier for chemotherapy.
The blood-brain barrier, a microscopic structure, shields the brain from most circulating drugs. As a result, the repertoire of drugs that can be used to treat brain disease is limited. Previously, the most potent chemotherapy drugs, paclitaxel and carboplatin, were not used in treating the deadly brain cancer glioblastoma because they could not permeate the blood-brain barrier to reach the aggressive tumor. Paclitaxel injected directly into the brain can result in brain irritation and meningitis. Temozolomide, the current glioblastoma chemotherapy, crosses the blood-brain barrier, but is a weak drug. No effective glioblastoma treatment currently exists.
The scientists discovered that ultrasonication -- the application of sound energy -- renders the brain permeable to drugs circulating in the bloodstream, but only for a limited time. The ultrasound and microbubble-based opening of the blood-brain barrier is transient, with most of the blood-brain barrier integrity restored within one hour following this procedure in humans. These findings allow optimization of the ultrasound activation and drug delivery sequence to maximize drug penetration into the brain.
The researchers were also able to quantify the effect of the ultrasound-based blood-brain barrier opening, measuring an approximately four- to six-fold increase in paclitaxel and carboplatin concentrations in the human brain.
To be effective, this approach requires coverage of a large region of the brain adjacent to the cavity remaining in the brain after glioblastoma tumor removal. Fortunately, the researchers found that using a skull-implantable grid of nine ultrasound emitters, designed by French biotech company Carthera, opens the blood-brain barrier in a sufficiently large volume of brain, nine times larger than the initial device.
In the phase I clinical trial, patients underwent surgery for tumor resection and implantation of the ultrasound device. They started treatment a few weeks after the implantation. An ongoing phase II clinical trial for patients with recurrent glioblastoma is now underway. Participants receive a two-drug combination of paclitaxel and carboplatin -- a combination also used to treat other cancers -- delivered to their brain via the ultrasound technique to investigate whether this treatment prolongs their survival.
The four-minute procedure to open the blood-brain barrier is performed with the patients awake; they are released after a few hours. The process is repeated with an escalated dose of paclitaxel delivered every three weeks. The treatment appears to be safe and well-tolerated, with some patients receiving up to six treatment cycles.
"While we have focused on brain cancer, this opens the door to investigate novel drug-based treatments for millions of patients who suffer from various brain diseases," Dr. Adam Sonabend, lead investigator and a Northwestern Medicine neurosurgeon, said in a statement. "This is potentially a huge advance for glioblastoma patients."
Copyright © 2023 scienceboard.net