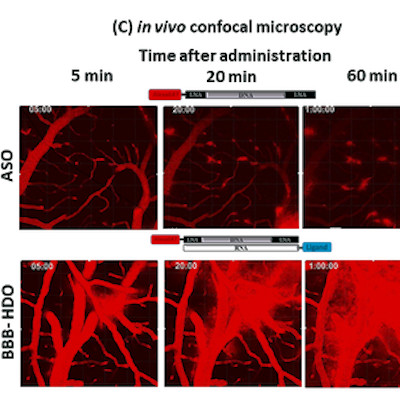
October 11, 2022 -- Investigators from Brigham and Women’s Hospital say the rational design of adeno-associated viruses (AAVs) has generated gene therapy delivery vectors that are better at crossing the blood-brain barrier to treat diseases of the central nervous system (CNS).
Of all AAVs, adeno-associated virus serotype 9 (AAV9) has shown the most potential to cross the barrier, which keeps toxins out of the brain, but even it is suboptimal for many therapeutic applications in the CNS. The limitations of existing gene therapy vectors are preventing researchers from turning knowledge of the genetic basis of diseases of the CNS into effective treatments.
However, in a study published October 10 in the journal Nature Biomedical Engineering, investigators from Brigham and Women's Hospital describe their work to improve on the existing gene therapy delivery vehicles. The collaborators used AAV9 as the starting point and sought to improve it by adding cell-penetrating peptides, molecules that are known to cross biological membranes.
After collecting around 100 cell-penetrating peptides, the researchers began modifying AAVs to display the molecules. The work led to the identification of two AAV variants, AAV.CPP.16 and AAV.CPP.21, that appear to more readily cross the blood-brain barrier. Fengfeng Bei, PhD, of the Brigham's department of neurosurgery, explained the significance of the findings in a statement.
"Our study is exciting because it shows that we are one step closer to being able to deliver gene therapy across the blood-brain barrier in humans," said Bei, who is a co-founder and scientific advisor of Brave Bio, an AAV gene therapy startup. "Our findings demonstrate that AAVs could provide a valuable tool for developing systemic gene therapies against glioblastoma and other diseases where CNS delivery is required."
After systemic delivery, the variants showed sixfold to two hundred forty-ninefold improvements in transduction efficiencies to CNS cells across four mice strains. The improvement is versus the AAV9 parent vector. In the primate species cynomolgus macaques, the researchers saw a fivefold improvement compared to AAV9. The variants were able to enter CNS cells in young and adult macaques.
Studies in a mouse model of glioblastoma suggest the variants may open up opportunities to treat CNS diseases. The researchers used AAV9 variants to deliver antitumor payloads to mice with glioblastoma, a hard-to-treat brain cancer that may respond to gene therapy. Some researchers are looking at delivering the therapy locally but systemic administration of a vector that crosses the blood-brain barrier would be simpler.
Yulia Grishchuk, PhD, who leads a lab in the Center for Genomic Medicine at Massachusetts General Hospital, sees opportunities to use the vectors to treat disease. Grishchuk recently collaborated with Bei.
"New treatments are urgently needed for neurometabolic diseases, lysosomal storage diseases and other diseases that affect both CNS tissue and other tissues in the body," Grishchuk said. "What is exciting here is that this work could represent a way to treat a broad spectrum of CNS disorders that are hard to target with current treatment approaches."
Copyright © 2022 scienceboard.net