 Future Fields, Jenthera Therapeutics collaborate to develop novel protein for the delivery of cancer biologics
Future Fields, Jenthera Therapeutics collaborate to develop novel protein for the delivery of cancer biologics
Biotech firm Future Fields and gene-editing firm Jenthera Therapeutics this week announced a collaboration focused on the manufacturing of a first-of-its-kind cancer-fighting protein. Read More
 Conductive, jelly-like material opens door to safer, more durable medical implants
Conductive, jelly-like material opens door to safer, more durable medical implants
Engineers have developed a jelly-like material that conducts electricity for use as an alternative to metal electrodes in medical implants. Read More
 New energy-generation methods could enable creation of entirely new organisms
New energy-generation methods could enable creation of entirely new organisms
Developing biologically realistic energy-generation methods that mimic natural processes could pave the way for the creation of entirely new organisms or biomaterials, according to a study published on Tuesday in Biophysics Reviews. Read More
 Beckman Coulter, Sciex collaborate on high-throughput screening workflows
Beckman Coulter, Sciex collaborate on high-throughput screening workflows
Life science analytical technologies company Sciex on Monday announced a collaboration with Beckman Coulter Life Sciences to provide comprehensive workflows for high-throughput screening; high-throughput absorption, distribution, metabolism, and excretion (HT-ADME); and synthetic biology studies. Read More
 Data-driven modeling predicts hydrogel properties to accelerate biomedical research
Data-driven modeling predicts hydrogel properties to accelerate biomedical research
Researchers have developed a data-driven modeling approach for predicting the properties of injected hydrogel blocks. Read More
 Mixing cancer drugs in nanoparticle combinations improves efficacy in mice
Mixing cancer drugs in nanoparticle combinations improves efficacy in mice
Using polymer nanoparticles to carry multiple cancer drugs achieves better outcomes than administering the combination of therapeutics without a delivery vehicle, according to preclinical research published on 26 January in Nature Nanotechnology. Read More
 Shrinking hydrogels enlarge nanofabrication options
Shrinking hydrogels enlarge nanofabrication options
Carnegie Mellon University and Chinese University of Hong Kong researchers are collaborating to reduce the size of printable nanodevices. Their work, published December 22 in Science, may facilitate the design and manufacture of sophisticated nanodevices. Read More
 Creating eye tissue using 3D bioprinting
Creating eye tissue using 3D bioprinting
National Eye Institute (NEI) scientists have produced viable eye tissue using patient stem cells and 3D bioprinting. Their research, published on Thursday in Nature Methods, advances the understanding of blinding eye diseases and provides a model for studying their genesis. Read More
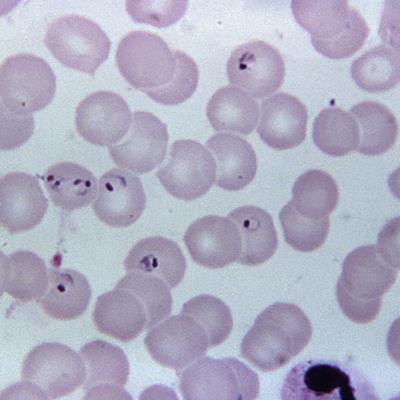 Scientists manufacture malaria sporozoites without mosquitoes
Scientists manufacture malaria sporozoites without mosquitoes
Sanaria scientists have manufactured Plasmodium falciparum (Pf) sporozoites (SPZ) in vitro, enabling the critical, first breakthrough steps to scale up manufacturing of the PfSPZ malaria vaccine. Read More
 Shenandoah expands cytokine, growth factors portfolio for cell and gene therapies
Shenandoah expands cytokine, growth factors portfolio for cell and gene therapies
Shenandoah Biotechnology, a Fujifilm Irvine Scientific company, has announced the expansion of its CTGrade portfolio of cytokine and growth factors for cell and gene therapies that are manufactured following current good manufacturing practices. Read More
Conferences
Member Rewards
Earn points for contributing to market research. Redeem your points for merchandise, travel, or even to help your favorite charity.
Research Topics
Interact with an engaged, global community of your peers who come together to discuss their work and opportunities.
Connect
Tweets by @ScienceBoard






