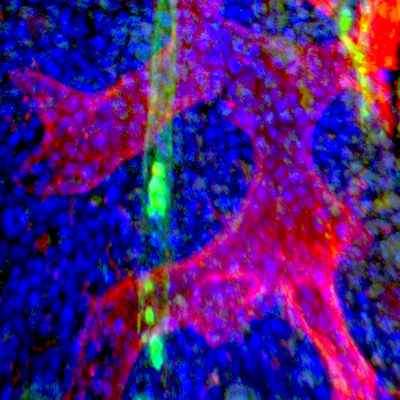
 Single-cell RNAseq differentiates cancer stem cells from healthy stem cells
Single-cell RNAseq differentiates cancer stem cells from healthy stem cells
Researchers have described a new single-cell RNA-sequencing approach, leveraging genomic and mitochondrial DNA mutations, that has the potential to differentiate normal stem cells from cancer stem cells. The article detailing the approach was published in Nature Communications on March 1. Read More
 Janssen COVID-19 vaccine becomes 3rd authorized in U.S.
Janssen COVID-19 vaccine becomes 3rd authorized in U.S.
Janssen Biotech's COVID-19 vaccine has received an emergency use authorization from the U.S. Food and Drug Administration. The move allows the vaccine to be distributed in the U.S. for the prevention of COVID-19 caused by SARS-CoV-2 in individuals 18 years of age or older. Read More
 FDA committee gives nod to Janssen COVID-19 vaccine
FDA committee gives nod to Janssen COVID-19 vaccine
A single-shot COVID-19 vaccine from Johnson & Johnson's Janssen Biotech subsidiary has received a positive recommendation from a U.S. Food and Drug Administration (FDA) advisory committee. Should the FDA grant the product emergency use authorization, the vaccine candidate will become the third to be made available in the U.S. Read More
 New open-access toolkit helps research labs study SARS-CoV-2
New open-access toolkit helps research labs study SARS-CoV-2
One of the most important factors in fighting the COVID-19 pandemic is large-scale scientific collaborations and knowledge sharing. One international group of researchers has made its simple, robust toolkit available to laboratories around the globe that are unaccustomed to working with coronaviruses. The details can be found in a PLOS Biology paper published on February 25. Read More
 Clinical pipeline promises effective 2nd-generation COVID-19 vaccines
Clinical pipeline promises effective 2nd-generation COVID-19 vaccines
Despite the small number of COVID-19 vaccines that have been granted emergency use authorizations by the U.S. Food and Drug Administration to date, many biopharmaceutical companies are continuing to push new vaccine candidates toward regulatory approval. The slow rollout of approved vaccines has created the need for second-generation products with the potential to accelerate the world's return to something approaching normalcy. Read More
 Game theory reveals how SARS-CoV-2 tricks human cells
Game theory reveals how SARS-CoV-2 tricks human cells
Researchers have applied game theory in an effort to understand how SARS-CoV-2 mimics host proteins to support its own replication. The work, published in Royal Society Interface on February 24, applied a type of game theory on how information is signaled to reveal how the virus tries to trick human cells from attacking it. Read More
 Automated flow cytometry can expedite the discovery and development of next-gen drugs
Automated flow cytometry can expedite the discovery and development of next-gen drugs
Automated flow cytometry workflows outperform manual gated techniques by reducing variability among samples, while still achieving a high degree of accuracy. Automated workflows offer a number of advantages to pharmaceutical companies that can integrate them to accelerate drug discovery and development. Read More
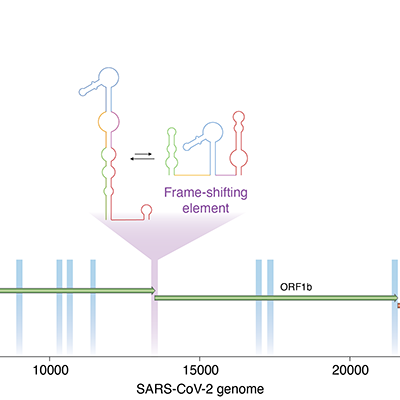 New technique detects unique folding patterns in RNA of SARS-CoV-2
New technique detects unique folding patterns in RNA of SARS-CoV-2
Scientists have developed a new technique for determining alternative structural RNA shapes of the SARS-CoV-2 virus. These self-regulatory segments of RNA, called switches, could serve as potential antiviral drug targets, according to a communication published in Nature Methods on February 22. Read More
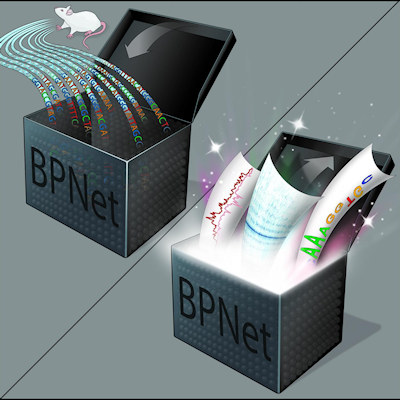 AI uncovers the genome's hidden regulatory code
AI uncovers the genome's hidden regulatory code
A neural network trained on high-resolution maps of protein-DNA interactions can uncover how these sequences are organized to regulate genes, revealing a hidden regulatory code. Findings from use of the artificial intelligence (AI) model were published in Nature Genetics on February 18. Read More
 The next generation of COVID-19 vaccines: Gallo on next steps
The next generation of COVID-19 vaccines: Gallo on next steps
What will the next generation of COVID-19 vaccines look like? In the first of a two-part series, The Science Advisory Board spoke with pioneering virology researcher Dr. Robert Gallo to get his thoughts on what a next-generation COVID-19 vaccine might look like, and which candidates are in the running for marketing authorization. Read More
Member Rewards
Earn points for contributing to market research. Redeem your points for merchandise, travel, or even to help your favorite charity.
Research Topics
Interact with an engaged, global community of your peers who come together to discuss their work and opportunities.
Connect
Tweets by @ScienceBoard



