 FDA authorizes Pfizer's COVID-19 pill
FDA authorizes Pfizer's COVID-19 pill
The U.S. Food and Drug Administration (FDA) has issued an emergency use authorization for nirmatrelvir and ritonavir tablets (which are co-packaged for oral use and sold under the brand name Paxlovid) for the treatment of mild to moderate COVID-19 in adults and pediatric patients (age 12 and older) who have tested positive for SARS-CoV-2 and who are at high risk of developing severe COVID-19, including hospitalization or death. Read More
 Seqster pursues mission to bring patients to center of healthcare
Seqster pursues mission to bring patients to center of healthcare
Patient access to data is a critical mission for the healthcare industry, according to Ardy Arianpour, CEO and co-founder of Seqster, who recently spoke with ScienceBoard Editor in Chief, Samantha Black, PhD. The company is focused on developing much-needed data interoperability tools for the healthcare sector. Read More
 Twist Bioscience launches 96-plex library prep kit
Twist Bioscience launches 96-plex library prep kit
Twist Bioscience has released the Twist 96-Plex Library Prep Kit, a high-performance, cost-effective preparation kit for next-generation sequencing. Read More
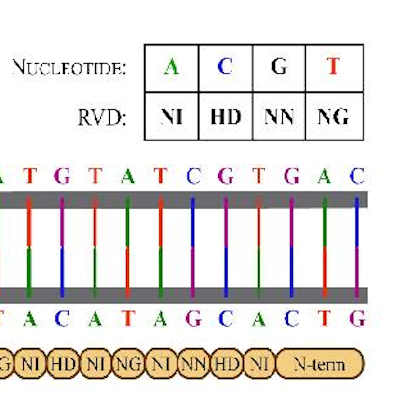 Cytovia expands partnership with Cellectis into China
Cytovia expands partnership with Cellectis into China
Cytovia Therapeutics and Cellectis have expanded their partnership on transcription activatorlike effector nuclease (TALEN) gene-edited NK cells derived from induced pluripotent stem cells (iPSCs) to allow for a broader collaboration in China. Read More
 EpiCypher secures co-licensing agreement for foundational chromatin technology
EpiCypher secures co-licensing agreement for foundational chromatin technology
EpiCypher has co-exclusively licensed cleavage under targets and release using nuclease and cleavage under targets and tagmentation technology from the Fred Hutchinson Cancer Research Center. The agreement strengthens EpiCypher's intellectual property to include both research-use-only and diagnostic applications. Read More
 New gene writing tech shows promise for large DNA insertions
New gene writing tech shows promise for large DNA insertions
An international team has developed a new gene writing technology called find cut-and-transfer that is able to make large gene insertions. The approach, published in Nature Communications on December 3, has potential gene editing and gene therapy clinical applications in patients with genetic and oncological diseases who have few treatment options. Read More
 Roche automates next-gen sequencing sample prep
Roche automates next-gen sequencing sample prep
Roche announced the launch of the Avenio Edge System, a core component of Roche's strategy to advance sequencing technologies. The system is built on Roche's foundational capabilities to deliver a fully automated, integrated sequencing solution. Read More
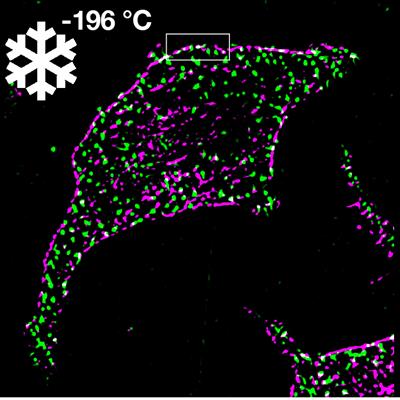 Cool imaging measures cells in motion
Cool imaging measures cells in motion
A new method, which involves cooling living cells at extremely fast speeds, leads to unprecedented preservation of cellular biomolecules in their natural arrangement at the moment of arrest. Details of the technique were published in Science Advances on December 10. Read More
 2021 Life Science Industry Award winners announced
2021 Life Science Industry Award winners announced
New technologies make advancements in life science research possible, such as the development of drugs, therapeutics, and molecular diagnostics. This year's winners represent a cross section of major laboratory equipment and consumable providers. Read More
 COVID-19-related sales remain strong for lab tool firms in 2021
COVID-19-related sales remain strong for lab tool firms in 2021
Laboratory tool companies have been among the biggest beneficiaries of U.S. government COVID-19-related spending on coronavirus testing and vaccine R&D and manufacturing. COVID-19-related sales have propelled sales at Thermo Fisher Scientific, Danaher, and PerkinElmer, already three of the world's largest scientific instrument and reagent providers. Read More
Member Rewards
Earn points for contributing to market research. Redeem your points for merchandise, travel, or even to help your favorite charity.
Research Topics
Interact with an engaged, global community of your peers who come together to discuss their work and opportunities.
Connect
Tweets by @ScienceBoard



