April 24, 2023 -- Researchers have developed a specialized printer to generate vaccine-filled microneedle skin patches that can be stored long-term without refrigeration. Their research, published Monday in Nature Biotechnology, may eventually bring more vaccines to more people.
Getting vaccines to people who need them can be challenging. Vaccines requiring cold storage are difficult to send to remote areas or store in places lacking refrigeration. Furthermore, traditional injectable vaccines require syringes, needles, and trained healthcare professionals to administer them.
The research team instead sought a way to produce vaccines on demand via a mobile printer that could produce hundreds of shelf-stable doses onsite each day. Such printers could be shipped to remote villages, refugee camps, or military bases, enabling the rapid vaccination of large numbers of people.
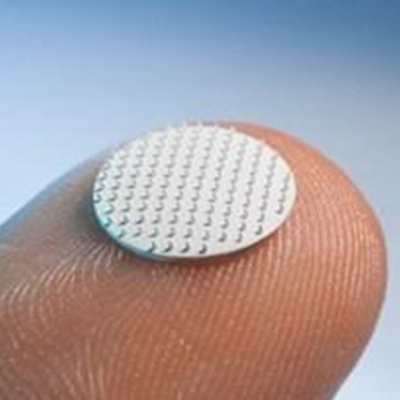
The COVID-19 pandemic motivated the researchers to incorporate RNA vaccines into their vision. They investigated a novel vaccine delivery system based on printable thumbnail-sized skin patches containing hundreds of microneedles, each containing vaccine. The microneedles are made with a 50/50 combination of polyvinylpyrrolidone and polyvinyl alcohol, providing the optimal combination of stiffness and stability. The ink in the microneedles includes RNA vaccine molecules encapsulated in lipid nanoparticles, which keep them stable for long periods of time.
Inside the printer, a robotic arm injects the ink into microneedle molds. A vacuum chamber below the mold sucks the ink down, ensuring that it reaches the tips of the needles. When the patch is applied, the needle tips dissolve under the skin, releasing the vaccine without requiring an injection.
To test the vaccines' stability, the researchers first created an ink containing RNA that encodes luciferase, a fluorescent protein. The printed patches were stored at either 4° C, 25° C (room temperature), or 37° C for varying lengths of time up to six months. When applied to mice, all patches induced a strong fluorescent response. By contrast, the fluorescent response produced via traditional intramuscular injection declined with longer storage times above room temperature.
To test the COVID-19 microneedle vaccine's efficacy, the researchers vaccinated mice with two doses of the RNA vaccine four weeks apart. Mice vaccinated with microneedle patches had similar antibody responses as mice vaccinated with traditional injected vaccines. Significantly, mice vaccinated with microneedle patches stored at room temperature for up to three months had the same strong antibody response.
The researchers said that the ink can potentially contain a variety of vaccines or drugs, allowing for flexibility in the medicines delivered. They plan to adapt the process for other medicines, including polio, measles, and rubella vaccines.
"If, for example, there was an Ebola outbreak in a particular region, one could ship a few of these printers there and vaccinate the people in that location," Ana Jaklenec, senior author and a Massachusetts Institute of Technology research scientist, said in a statement. "We could someday have on-demand vaccine production."
Copyright © 2023 scienceboard.net