August 27, 2020 -- Biopharmaceutical firm Verndari has secured funding from the Biomedical Advanced Research and Development Authority (BARDA), a U.S. federal health agency, to support development of its VaxiPatch microneedle dermal patch vaccine technology.
The funding is administered through BARDA's Division of Research, Innovation, and Ventures (DRIVE), and consists of a $1 million cost-sharing arrangement under which BARDA will provide 68% of the total.

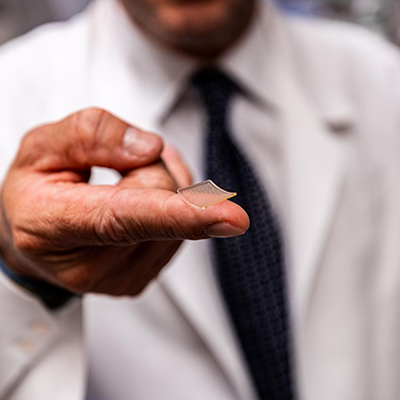
VaxiPatch is a single-dose vaccination kit that administers vaccines through the skin, according to the firm. It does not require refrigeration. VaxiPatch is currently in preclinical testing at the University of California, Davis, for delivering a vaccine for SARS-CoV-2, Verndari said.
The company is in discussion with the U.S. Food and Drug Administration regarding an investigational new drug submission for VaxiPatch.
Copyright © 2020 scienceboard.net