 Anti-herpes drug weakens antibiotic-resistant bacteria
Anti-herpes drug weakens antibiotic-resistant bacteria
November 7, 2022 -- An anti-herpes drug discovered in the 1960s weakens the protective surface of an antibiotic-resistant bacteria and makes it easier for the immune cells to eliminate the bacteria, researchers from Switzerland found. Read More
 Researchers develop novel platform to improve immunotherapy
Researchers develop novel platform to improve immunotherapy
November 4, 2022 -- Researchers have discovered a novel pathway very early during in vitro differentiation that supports the emergence of T cells and natural killer cells from human induced pluripotent stem cells. Read More
 Study finds certain lymphoid cells not superfluous, offers insight into inflammatory diseases
Study finds certain lymphoid cells not superfluous, offers insight into inflammatory diseases
November 3, 2022 -- A new study reveals group 2 innate lymphoid cells are not redundant and in fact are essential for protecting the skin, gastrointestinal tract, airways, and other barrier tissues from parasitic infections as well as damage associated with allergic inflammation and asthma. Read More
 Vitamin C boosts gene activation in dendritic cells
Vitamin C boosts gene activation in dendritic cells
November 3, 2022 -- Vitamin C can improve the immunogenic properties of dendritic cells in vitro and may hold the key to improving efficacy of anticancer cell therapies, according to a team from the Epigenetics and Immune Disease Lab at the Josep Carreras Leukaemia Research Institute in Barcelona, Spain. Read More
 Advanced nanoparticles help fight difficult cancers
Advanced nanoparticles help fight difficult cancers
October 28, 2022 -- University of Chicago researchers are using tiny molecules called advanced nanoparticles to deliver compounds that suppress tumor growth and metastasis. The study, published October 26 in the journal Nature Nanotechnology, holds promise for treating difficult cancers. Read More
 Enzyme suppression boosts immune response in cell cultures and mice
Enzyme suppression boosts immune response in cell cultures and mice
October 11, 2022 -- Researchers from The Ohio State University have identified an enzyme-inhibiting strategy that may promote a strong enough immune response to stop viruses in their infectious tracks. Read More
 Experimental treatment relieves asthma without weakening flu defenses in mice
Experimental treatment relieves asthma without weakening flu defenses in mice
October 10, 2022 -- A new NYU study found that blocking the action of calcium signals in immune cells suppressed asthma in mice without compromising their defenses against flu viruses. Read More
 A monkey virus could transfer to humans, study finds
A monkey virus could transfer to humans, study finds
October 3, 2022 -- University of Colorado Boulder researchers are urging vigilance around an obscure family of viruses known to cause fatal Ebola-like symptoms in some monkeys. They said the virus is “poised for spillover” to humans, although that hasn’t happened yet. Read More
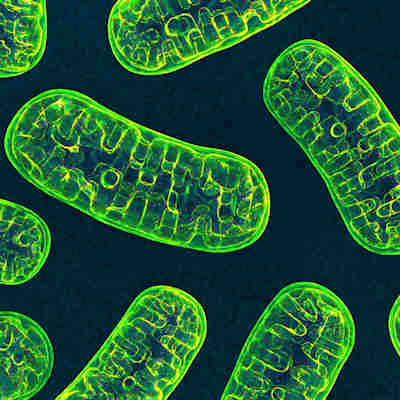 Mitochondria shape influences autoimmune disorders: study
Mitochondria shape influences autoimmune disorders: study
September 29, 2022 -- The shape and function of the immune system’s Th17 mitochondria play a key role in autoimmune and inflammatory disorders such as multiple sclerosis, new research suggests. Read More
 Scientists identify immune cell targets to fight chemo-resistant breast cancer
Scientists identify immune cell targets to fight chemo-resistant breast cancer
September 27, 2022 -- London-based scientists have identified certain immune cells that may potentially be targeted to develop immunotherapies that benefit patients with chemotherapy-resistant breast cancers. Read More
Conferences
Member Rewards
Earn points for contributing to market research. Redeem your points for merchandise, travel, or even to help your favorite charity.
Research Topics
Interact with an engaged, global community of your peers who come together to discuss their work and opportunities.
Connect
Tweets by @ScienceBoard



