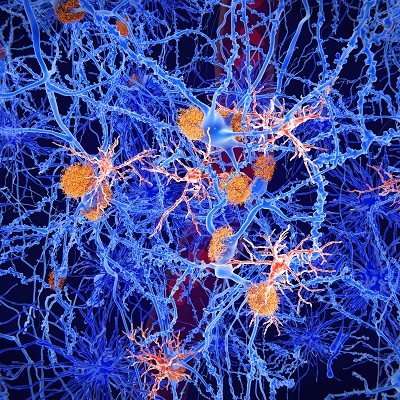
May 10, 2023 -- Researchers showed that tiny aggregates of amyloid beta protein, a naturally occurring substance associated with Alzheimer's disease progression, can float freely through brain tissue fluid, reaching important brain regions and disrupting local neuron functioning. The research, published Wednesday in the journal Neuron, also indicated that a newly-approved Alzheimer's disease treatment could neutralize these tiny aggregates, also known as protofibrils or oligomers.
Alzheimer's disease, which causes dementia, affects more than 50 million people worldwide. Previous research showed that Alzheimer's disease patients have abnormal build-ups of amyloid beta protein in the brain, which can disrupt neurotransmission. Currently, there is no cure for the disease.
Last January, the U.S. Food and Drug Administration (FDA) approved lecanemab, an antibody therapy for treating Alzheimer's disease, developed by Biogen and Eisai.
In phase III clinical trials, lecanemab slowed cognitive decline in patients with early Alzheimer's disease. Scientists suspect the drug's beneficial effect may be associated with its ability to bind and neutralize soluble amyloid beta protein aggregates. These tiny clumps of amyloid beta protein can form in the brain, then proceed to aggregate further into large amyloid plaques. Tiny aggregates can also break off and diffuse away from amyloid plaques that are already there. These free-floating aggregates can access important brain structures such as the hippocampus, which plays a major role in memory.
However, scientists have previously been unable to define a particular protofibril or oligomer that lecanemab binds to -- a detail important to patients and drug developers who want to know whether the aggregates that lecanemab binds to can reveal how the drug works.
The researchers successfully isolated the free-floating amyloid beta aggregates from the human brain by soaking postmortem brain tissues from typical Alzheimer's disease patients in saline solutions, then spinning the aqueous extracts at high speeds, a process called ultracentrifugation. Working collaboratively, the team determined the atomic structure of these tiny aggregates, down to the individual atom, to properly identify them.
Next, the team plans to investigate how these tiny amyloid beta aggregates travel through living animal brains and how the immune system responds to these toxic substances. Recent research has shown that the brain's immune system reaction to amyloid beta is a key component of Alzheimer's disease.
"If we can figure out exactly how these tiny, diffusible fibrils exert toxicity, then maybe the next Alzheimer's disease drugs can be better," said first author Andrew Stern, Brigham and Women's Hospital neurologist, in a statement.
"For the first time in human history, we have an agent that can actually treat people with Alzheimer's in a way that could slow their cognitive decline," corresponding author Dr. Dennis Selkoe, Harvard Medical School professor of neurologic diseases, said in a statement. "We've never been able to say those words until the last few months."
Copyright © 2023 scienceboard.net