November 6, 2019 -- A group of academic and industry researchers developed new Liver-Chips with four cell types found in the livers of rats, dogs, and humans. The idea was generated at Wyss Institute for Biologically Inspired Engineering at Harvard and then transferred to Emulate Inc., to provide real-time analysis of complex biochemical interactions and enhanced liver toxicity testing.
The results are presented in Science Translational Medicine on November 6.
Traditionally, drugs that enter clinical trials must first be tested in animals to ensure that they are safe before being administered to humans. Human liver toxicity is one of the primary reasons why drugs fail in clinical trials. Therefore, there must be discrepancies between the results produced from liver toxicity observed in animal models and the responses later seen in humans. A recent analysis of 150 drugs showed regulatory testing in animals could only predict 71% of toxicities in humans. The solution could lie with the new Liver-Chip technology.
"This is an important milestone in the effort to improve the drug discovery and development process that was achieved by leveraging the Wyss Institute's unique translational model, which allowed us to evaluate the promise of Organ-Chips both technically and commercially early on in the development of the technology," said corresponding author Geraldine A. Hamilton, PhD, President and Chief Scientific Officer of Emulate. "We are excited to see what advances our customers will be able to achieve using this Liver-Chip, and we are grateful for the opportunity to impact the drug discovery and development process and make a difference in the lives of patients."
This technology consists of a clear, flexible polymer about the size of a USB drive with parallel internal channels that are lined with living cells. The spatial arrangement of the channels and cell types more accurately recreates the tissue microenvironment of human organs in vivo and exhibits physiological responses and disease states that are similar to those that occur in humans. These Liver-Chips could help ensure that safe and effective therapeutics are identified sooner, and ineffective or toxic ones are rejected early in the drug development process.
The Liver-Chip system allowed researchers to use drug concentrations that bridged those measured in the plasma of nonclinical models or humans in the present study, and suggesting that the model has the potential to be used for human risk assessment. The model has the ability to measure mechanistic endpoints and biomarkers which makes it suitable for delineating pathways and mechanisms causing DILI.
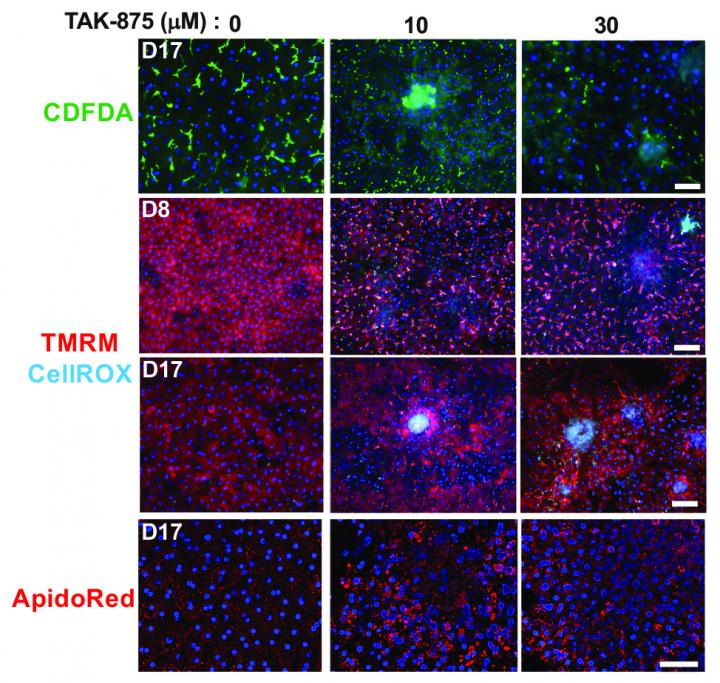
Human-specific sensitivities to toxicity by bosentan and FIAU were also confirmed in the human Liver-Chip compared to the companion animal Liver-Chip. Moreover, the researchers confirmed the mechanism of toxicity of JNJ-1 in the Liver-Chip model that has been observed in previous studies with genetically modified mice. They also found that a related compound, JNJ-2, which was previously determined as unsafe in animals, may not necessarily be toxic in humans. According to results from the Liver-Chip experiment, JNJ-2 caused harmful fibrosis in rat chips but did not have negative effects on human liver cells.
"This work represents a major achievement for the Organ Chip field because it shows the power of this technology to provide insight into human-relevant responses where current preclinical animal models often fail. This needs to be evaluated and confirmed by others, but if it is, then this could change the way drugs are developed around the world and help begin to reduce the numbers of animals that are used in drug development efforts worldwide," said co-author Don Ingber, who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and the Vascular Biology Program at Boston Children's Hospital, and Professor of Bioengineering at Harvard's John A. Paulson School of Engineering and Applied Sciences.
Many organ-on-a-chip systems including lung, intestine, brain, kidney, bone marrow have been developed at the Wyss Institute before being transferred to Emulated for commercialization. This has been accomplished through a number of collaborations industry and with grant support from the Defense Advanced Research Projects Agency (DARPA), Food and Drug Administration (FDA) and National Institutes of Health (NIH).
Do you have a unique perspective on your research related to pre-clinical drug discovery and development? Contact the editor today to learn more.
Copyright © 2019 scienceboard.net






