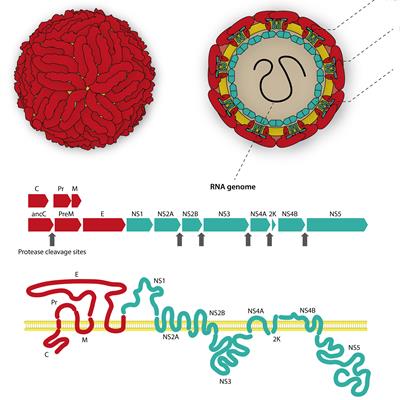
September 13, 2022 -- A genomics profiling method has revealed how the Zika virus infects human dendritic cells, pointing to a potential target for therapeutic suppression of the pathogen.
In a study published on September 12 in the journal Nature Communications, researchers from the La Jolla Institute (LJI) and University of California, San Diego described the creation of a genomics profiling method to enable the discrete analysis of Zika-infected versus neighboring, uninfected primary human cells. The researchers developed the method to answer a key question about how the virus subverts the function of dendritic cells.
"Dendritic cells are major cells of the innate immune system," Professor Sujan Shresta, PhD, a member of the LJI Center for Infectious Disease and Vaccine Research, said in a statement. "How is this virus so clever that it's able to establish infection in cells that would normally fight infections?"
The tool empowered the researchers to probe that question by increasing the sensitivity and specificity with which Zika-modulated pathways can be identified. Armed with the new method, the collaborators showed that infection increases the expression of genes enriched for lipid metabolism-related functions. The team also found that infection increases the recruitment of sterol regulatory element-binding protein (SREBP) transcription factors to lipid gene promoters.
The discovery of the role of SREBP suggested a way to fight back against the virus. After showing that SREBP2-dependent lipid gene transcription enhances the infection of human dendritic cells by Zika, the researchers speculated that the protein may have therapeutic applications.
To test the hypothesis, the team preincubated monocyte-derived dendritic cells with a molecule that suppresses SREBP processing. The molecule inhibited infection by 36% and 47%, depending on the strain of Zika virus tested. The treatment reduced the level of intracellular virus RNA by 8-fold, extracellular virus RNA by 86-fold, and the number of secreted infectious particles by 257-fold to 1000-fold, again depending on the viral strain.
Further studies showed knockdown of the SREBF2 gene, not SREBF1, inhibited Zika infection. The studies suggest small interfering RNA-mediated knockdown represents another way to tackle infection and indicate that SREBF2, rather than SREBF1, plays an important role in viral replication. Other researchers have found that SREBF2 is a key host factor in pathogens including SARS-CoV-2.
"Targeting host lipid metabolism could provide antiviral therapies for many important human pathogenic viruses that lack U.S. Food and Drug Administration-approved therapies. Understanding how distinct viruses manipulate host lipids will be critical for developing optimal lipid-targeting therapies," the researchers concluded.
The flavivirus family causes several diseases, including dengue, Japanese encephalitis, Zika, West Nile disease, and others. Shresta contends that the more knowledge generated about these viruses "the closer we are to a 'pan-flavivirus' inhibitor."
Copyright © 2022 scienceboard.net