July 27, 2018 -- Here at The Science Advisory Board we aim to keep ahead of the curve with the latest breakthroughs in the life sciences field. An exciting advancement to follow in this space is precision medicine. The NIH defines precision medicine as “an emerging approach for disease treatment and prevention that takes into account individual variability in genes, environment, and lifestyle for each person." Within the last week, the liquid biopsy has made headlines as a diagnostic test for patients with cancer.
On Friday March 16, 2018, the Centers for Medicare & Medicaid Services (CMS) took action to advance innovative personalized medicine for Medicare patients with cancer. CMS announced it has finalized a National Coverage Determination that covers diagnostic laboratory tests using Next Generation Sequencing (NGS) for patients with advanced cancer. This decision is likely to accelerate the adoption of NGS in clinical diagnostics from liquid biopsies.
According to the National Cancer Institute (NCI), approximately 40% of men and women will be diagnosed with cancer at some point during their lifetimes. Cancer is a disease of the DNA, in which complementary genetic mutations push a cell into a state of constant replication whilst avoiding the body’s natural defenses. Knowing the specific mutations within a cancer can help predict a drug’s effectiveness or suggest resistance. Following the discovery that tumors shed their DNA into the bloodstream and advances in DNA sequencing technology, the liquid biopsy has become an exciting new method for uncovering the genetic signatures of cancers.
The standard liquid biopsy tests a patient's blood serum for mutations in the circulating tumor DNA (ctDNA) or messenger RNA (mRNA), typically through real-time PCR, digital PCR, or next-generation sequencing (NGS). The non-invasive nature of this simple blood test is extremely appealing when compared to the tissue biopsy, which often requires surgery on already weakened patients. Within the past few years, countless testing laboratories have started to offer liquid biopsy genetic tests ranging from one to hundreds of genetic mutations assessed at a time. Mutations are not tested randomly, but instead carefully chosen for their association with the type of tumor and treatment options.
The ability of a physician to use genetic profiling to inform treatment decision-making is a form of precision medicine, a concept becoming highly prevalent in the field of oncology. The success of precision medicine relies on several factors: highly correlated biomarkers with precision drugs, reliable biomarker testing, knowledgeable physicians, and willing patient participants. In cancer, DNA mutations have become a popular biomarker due to the genetic origins of cancer and reliable testing methods. With more and more precision cancer drugs coming to market with corresponding genetic biomarkers, a liquid biopsy is an enticing approach for simple, non-invasive testing.
Momentum and adoption of liquid biopsies
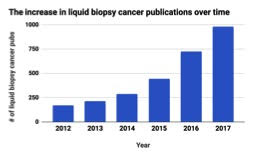
There is no denying that the field of oncology has embraced the liquid biopsy. According to PubMed, a search engine accessing life science literature, the number of publications that mention cancer and either ctDNA or liquid biopsies has exploded following 2014. Since the liquid biopsy is a relatively new term, it is not always used in scientific publications, however, the detection of ctDNA from blood plasma is considered a liquid biopsy and the terminology is used interchangeably. In 2017, there were almost 1000 cancer publications investigating liquid biopsies, a 300% increase when compared to about 250 in 2014.
Liquid biopsies are also a hot topic at the major cancer research meetings held by the American Association for Cancer Research (AACR) and American Society of Clinical Oncology (ASCO). At the 2017 AACR annual meeting, 236 abstracts mention liquid biopsy and 88 mention ctDNA. A similar amount of enthusiasm for the technique was seen at the 2017 ASCO annual meeting with 62 abstracts mentioning liquid biopsies and 161 abstracts mentioning ctDNA. At these meetings, leaders of the industry present their research and doctors from around the world listen. It is at meetings such as these where the word is spread about new and revolutionary technologies.
The most potent driver for the acceptance and use of liquid biopsies in clinical practice is completed clinical trials showing the data-backed benefits of using liquid biopsies on patient prognosis. According to clinicaltrials.gov, a registry of U.S. clinical trials, there have been 14 completed clinical trials mentioning liquid biopsies and 18 mentioning ctDNA. These are small numbers compared to the active clinical trials, 173 of which mention ctDNA and 61 mention liquid biopsies. These numbers illustrate the growth that is still in progress for liquid biopsy advancement and adoption.
In June of 2016, the FDA
approved the first liquid biopsy genetic test. The test is a blood-based companion diagnostic for the cancer drug Tarceva (erlotinib) called the cobas EGFR Mutation Test v2 from Roche Molecular Diagnostics. The test is used to detect epidermal growth factor receptor (EGFR) gene mutations in non-small cell lung cancer (NSCLC) patients using real-time PCR. Patients testing positive for certain mutations within the EGFR gene are eligible for treatment with Tarceva. However, if the test is negative for EGFR mutations, a tissue biopsy is recommended, as they are 10-15% more sensitive than liquid biopsies. The cobas EGFR Mutation Test v2 is still the only FDA-approved liquid biopsy test that analyses ctDNA, however, FDA approval is not a prerequisite for clinical use and doctors are not deterred from ordering liquid biopsy tests that don’t yet have FDA approval. It is understood by doctors that gaining FDA approval is a long and arduous process and can slow down the adoption of life-saving technologies.Another milestone for liquid biopsies occurred in January of 2018 when the National Comprehensive Cancer Network (NCCN) updated their guidelines on NSCLC to include the use of “plasma biopsies”, another name for liquid biopsies. The NCCN is an alliance of 27 cancer centers in the U.S., most of which are designated as comprehensive cancer centers by the NCI. Their guidelines are intended to assist in the decision-making process of individuals involved in cancer care and are widely adopted by U.S. oncologists. The inclusion of the “plasma biopsies” within their guidelines is a first for the NCCN and highlights the acceptance of the liquid biopsy into mainstream cancer care and the expectation for its use as standard practice.
One significant hurdle to the clinical use of liquid biopsies is the lack of reimbursement for the tests, especially those that evaluate large panels of genetic mutations since there may not be any direct evidence that some of the mutations tested are clinically actionable. Without reimbursement, patients end up responsible for the hefty bill and doctors are less likely to order the tests. In Friday’s breakthrough decision, the Centers for Medicare and Medicaid Service (CMS) has committed to the reimbursement of all FDA-approved or -cleared NGS cancer panels used as companion diagnostics.
“CMS believes when these tests are used as a companion diagnostic to identify patients with certain genetic mutations that may benefit from U.S. Food and Drug Administration (FDA)-approved treatments, these tests can assist patients and their oncologists in making more informed treatment decisions,” stated the CMS in a press release issued regarding their finalized National Coverage Determination (NCD). “Additionally, when a known cancer mutation cannot be matched to a treatment then results from the diagnostic lab test using NGS can help determine a patient’s candidacy for cancer clinical trials.”
Even more encouraging than the CMS’s coverage promise for FDA-approved NGS cancer panels, is the removal of a statement in the draft of the NCD stating that non-FDA-approved NGS panels would need to apply for reimbursement through the coverage with evidence development (CED) process, which allows Medicare to provide conditional coverage while additional clinical or scientific information is collected. After receiving hundreds of letters and comments from the community, the CMS conceded that evidence has already been or is currently being developed for non-FDA-approved NGS panels, exempting them from having to go through the CED process, which could delay coverage. Instead, NGS panels not approved by the FDA can apply for coverage through the regional Medicare Administrative Contractors.
Use of liquid biopsies in practice
The liquid biopsy was developed as an alternative to the tissue biopsy as a non-invasive and relatively fast way to test for genetic aberrations to inform treatment decisions. The tissue biopsy, though, is still the gold standard as a cancer diagnostic for several reasons. Depending on the mutation being tested for, liquid biopsies can range in sensitivity from 60 to 80%, leading to a relatively high rate of false negatives in some cases. Tissue biopsies, on the other hand, while not perfect, have genetic mutation detection sensitivities between 80 and 90%, making tissue a more reliable material for testing.
Another advantage of tissue biopsies is that they can give a physician more information about the state of a cancer than a liquid biopsy. For example, a tissue biopsy is needed to analyze the morphological features of the tumor, frequently necessary for a proper diagnosis. Additionally, several non-sequencing methods for detection of mutations, such as fluorescence in situ hybridization (FISH) and immunohistochemistry (IHC), can be used on a tissue biopsy, minimizing the reliance on sequencing and confirming their presence within the tumor.
“No one has come out and said, nor is there compelling data that liquid biopsy should replace tissue biopsy,” said Dr. Wafik El-Deiry, physician and researcher at Fox Chase Cancer Center in Philadelphia, PA. “The likelihood is that they will complement each other because they can report on different things. Tissue biopsy reflects the actual tissue at the location in which it was sampled, whereas liquid biopsy gets its components from multiple metastases.”
Liquid biopsies sample from the blood, capturing DNA from heterogeneous tumors and metastases located in different parts of the body that could contain different mutations from the main tumor. Used concurrently with a tissue biopsy during initial diagnosis, the liquid biopsy could give a more comprehensive picture of the patient’s cancer. Although an appealing assertion, in practice, this is not how liquid biopsies are most commonly used since there is not enough evidence to show that using liquid biopsies in this way has a significant prognostic benefit to patients.
The bulk of liquid biopsy tests ordered by community oncologists are to assess mutational changes in cancer that may be causing resistance to the patient’s current therapy. Just as all life evolves by adapting to a particular environment through genetic changes over time, cancers can evolve within the body, giving them resistance to drugs by acquiring new mutations. Once it’s determined that a patient’s tumor is not responding to treatment, on that same day, a liquid biopsy test can be ordered and a blood sample shipped to the testing laboratory. Results from a liquid biopsy genetic analysis can take anywhere from 3-9 days depending on the laboratory and test being ordered. Alternatively, in the case of solid tumors, scheduling a tissue biopsy can take weeks, plus another few days to get results back.
“What I’ll do, is have a patient scheduled through interventional radiology and biopsy in 10 to 14 days, but send off a plasma test immediately, and if the plasma test is positive, you can abort the surgery,” said Dr. H. Jack West, medical oncologist and Director of the thoracic oncology program at The Swedish Cancer Institute in Seattle, WA.
A positive liquid biopsy test result means that there are one or more new mutations detected in the resistant cancer that will guide the next steps in treatment. The next step could be treatment with a precision drug, or enrollment in a clinical trial. If the liquid biopsy test result is negative, a tissue biopsy would still need to be performed, as tissue biopsies are more sensitive and may reveal mutations not picked up by the liquid biopsy.
Nevertheless, if mutations are detected in either liquid or tissue biopsies, oncologists must make a decision on how best to treat the patient based on this new information. To make informed decisions, oncologists partially rely on reports provided by the testing laboratory that describe details about pathology of each positive mutation, what FDA-approved drugs are available that work with the mutation, and what clinical trials are available that use the mutation as a biomarker. Keeping up with the medical literature is a critical piece to the process of precision medicine and can be difficult to do within the rapidly changing industry.
For most oncologists, ordering liquid biopsy tests from independent testing laboratories is standard, as there is no in-house option
“For now, liquid biopsies will be a send out test,” said Dr. Milan Sheth, oncologist at the Todd Cancer Pavilion Specialty Clinic in Long Beach, CA. “The bigger university centers with more volume might look into buying the equipment, but community hospitals probably wouldn’t have the funds, volume, or expertise of running the assays.”
Most liquid biopsy mutational testing is performed via NGS, which is a fairly complex technology that requires precision handling of reagents and bioinformatic analysis. A standard medical laboratory is not equipped to adopt the technique and would need to make a significant investment in equipment and personnel. Development of the sample processing methods, bioinformatic pipeline, and regular validation testing also add to the cost of setup and maintenance making in-house NGS testing unreasonable for most community hospitals.
If the decision is made to incorporate NGS testing into an in-house laboratory, results could potentially arrive a few days faster than a send-out test, which would allow oncologists to start a new treatment path more quickly. However, the timeline to results would depend on the demands of the lab and not be guaranteed. Another potential benefit of in-house testing would be control over the process, making the test more consistent within a particular hospital network. Consistency of test results means consistency in treatment programs among patients and less risk. Recently, disagreement among popular NGS panels has been a topic spotlighted in the public.
In December of 2017, a paper published in JAMA Oncology tested the concordance of two commercially available liquid biopsy NGS panel tests: the Guardant360 from Guardant Health Inc., and PlasmaSELECT-R64 from Personal Genome Diagnostics. The authors sent identical samples from 40 patients to each company and compared the results. Only three of the results matched between companies sending up bright red flags within the industry. The authors did not use another method to evaluate mutations within the samples, so there is no way of knowing if the discrepancy between tests occurred due to false negatives or false positives.
Though appalling to some in the industry and public, the results from this study has not deterred doctors from using liquid biopsies. To mitigate risks of incorrect or incomplete results from liquid biopsies, doctors consistently use the same companies to order tests from and rarely use liquid biopsies alone to make treatment decisions. It is also well known that liquid biopsies have a relatively high false negative rate, so these results were somewhat expected.
Oncologists take on the responsibility for deciding which tests to order and how best to interpret and use the results. Patients are largely unaware of liquid biopsies and are perfectly willing to follow doctor’s orders when a simple blood draw is all that’s needed for the test. The potential for liquid biopsies is immense, but doctors should be aware of the limitations and proceed with caution when incorporating liquid biopsies into their standard-of-care.
What do you think?
What are your thoughts on the emerging precision medicine technologies? Where do you predict medicine will be in the next 5 years? Start the discussion below in Disqus!
Special thanks to Kristen Slawinski, Ph.D. for her assistance with this article.
Copyright © 2018 scienceboard.net






