September 28, 2020 -- Bioray Laboratories posted interim results from clinical trials of its nonviral programmed cell death 1 (PD1) specific targeted chimeric antigen receptor (CAR) T therapy for relapsed/refractory B-cell non-Hodgkin lymphoma.
The results of the clinical trial program were published in medRxiv on September 23 and showed partial remission in 4 of 15 patients after receiving the one-month treatment, and complete remission in two patients after three months of treatment. Importantly, there were no serious adverse effects related to the CAR T therapy. The company is currently evaluating this CAR T product in several other clinical trials.
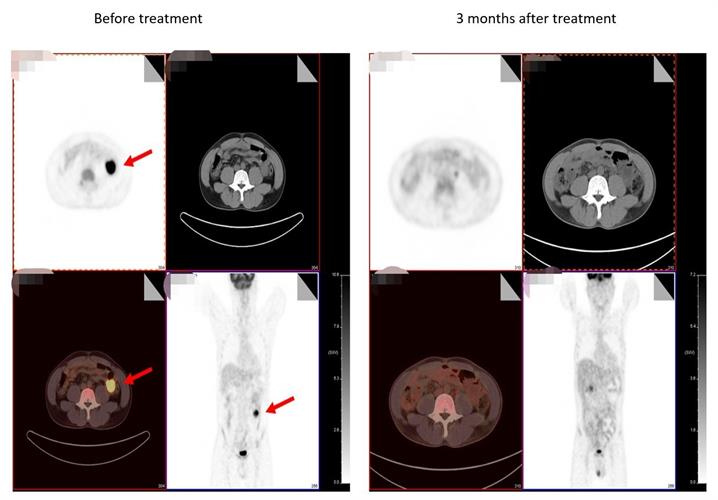
The company developed the PD1 specific targeted CD19-CART using its Quikin CART platform, which precisely inserts CAR cassette into PD1 locus without using a virus. The product combines PD1 immune checkpoint inhibition with CART anti-tumor activity to generate both anti-PD1 immunotherapy and CAR T therapy. Other advantages of Quikin technology include a simpler process, fewer production links, shorter preparation times, and higher product uniformity.
In addition to evaluating the nonviral targeting CART products for lymphoma, Bioray Laboratories is conducting research using the product for the treatment of solid tumors.
Copyright © 2020 scienceboard.net