May 29, 2020 -- Key immunological factors underlying the clinical stages of COVID-19 that could be targeted by therapeutic drugs were outlined in a review by researchers from the U.S. Food and Drug Administration (FDA). The article was published in Frontiers in Immunology on May 29.
Since emerging in late 2019, SARS-CoV-2 has spread around the world. Although SARS-CoV-2 belongs to the same family of coronaviruses as SARS-CoV and MERS-CoV, the epidemiology of COVID-19 (the disease caused by SARS-CoV-2) differs from previous coronavirus-related diseases. In particular, SARS-CoV-2 has a greater ability to be transmitted among populations and infect a larger number of individuals.
Understanding the specific characteristics of SARS-CoV-2 infection, as well as the host response to the virus, is critical to making informed decisions regarding strategies to combat the virus, especially in stage-specific manifestations.
The researchers reviewed possible immunological events of COVID-19 that cause a shift from protective antiviral immunity to hyperinflammatory responses. However, they noted that there is currently a lack of animal models and experimental data, so they integrated available COVID-19 clinical reports with scientific literature pertaining to SARS-CoV and MERS-CoV infections and cytokine storms.
Viral entry of SARS-CoV-2 is enabled by binding to angiotensin-converting enzyme 2 (ACE2), which is expressed on cell surfaces via the SARS-CoV-2 spike (S) protein. This penetrates host cell activation and catalytic activity of the cellular transmembrane protease serine 2 (TMPS2) (encoded by the gene TMPRSS2). The authors suggested that the interaction of the viral S protein with ACE2 may result in dysregulation of the renin-angiotensin system, thus lowering ACE2 levels and increasing angiotensin II (similar to cytokine storms).
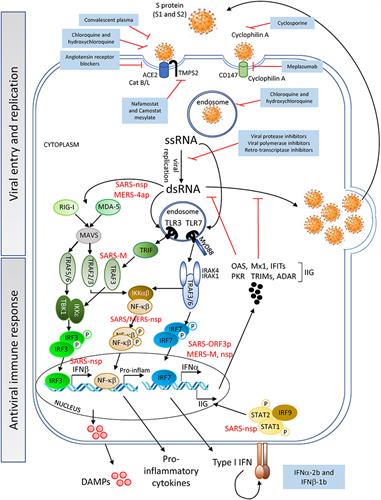
After viral entry, the SARS-CoV-2 positive-sense single-stranded (ss) RNA genome initiates its replication using self and host proteins. Both genomic ssRNA and double-stranded (ds) RNA intermediate molecules can be recognized by the host immune system.
Replication of SARS-CoV-2 could use mechanisms similar to those employed by SARS-CoV or MERS-CoV to evade the early steps of the host innate antiviral response. If this is true, then it is probable that SARS-CoV-2 proteins interfere with the activation of the type I interferon (IFN) pathway.
Indeed, SARS-CoV-2 has been shown to suppress type I and III IFN genes in vitro, in animal models, and in COVID-19 patients. The researchers noted that COVID-19-related inflammatory responses could also be induced by the dysregulation of the complement system, a critical component of the host innate immunity.
The researchers suggested that a cytokine storm develops after an initial immune response by the induction of cytokines by viral RNA activation of the innate immune system and the release of damage-associated molecular patterns (DAMPs) by apoptotic and necrotic cells. The response to SARS-CoV-2 results in an amplification of inflammation with the release of high levels of the proinflammatory cytokines interleukin-6 (IL-6), IL-18, tumor necrosis factor (TNF), and IL-1-beta by macrophages, and of IFN-gamma by natural killer (NK) cells.
The hyperinflammatory environment can potentially recruit and hyperactivate T cells, which in turn contribute to the inflammatory damage of tissues. This could happen at the infection site in the lungs. Alternatively, infiltration of T cells could be facilitated by upregulation of adhesion molecules by lung endothelial cells.
Regardless of mechanism, T and NK cells acquire an exhausted phenotype during COVID-19 disease progression. Both cell types show dysfunctionality after being fully active, including in their capacity to make proinflammatory cytokines.
Using lessons learned to inform therapeutic development
Repurposed therapeutics currently being used to treat inflammatory conditions are derived from conditions that share some features with COVID-19. These drugs are used in a variety of ways including as monotherapies and combination therapies. The data generated from clinical observations often lack adequate control arms, lack standardized reporting, and are derived from a heterogeneous patient population.
According to the researchers, the most challenging component of COVID-19 is controlling the detrimental inflammation caused by SARS-CoV-2 without compromising protective immune responses. They suggested that the use of drugs and timing of intervention (stage of the disease) aligned with mechanisms of action will help physicians make informed decisions and point to potential therapeutic and vaccine developments.
"Approaches to therapy in the early stage of the disease will differ from those in its severe late stage," said senior author Montserrat Puig, PhD, a biologist with the FDA. "As the results of clinical trials become available, it may become increasingly clear that there is likely no single magic bullet to resolve the disease but a combination of several interventions that target different key factors of COVID-19 may well be required."
Therefore, drugs for each stage of the disease will require unique solutions. These include drugs that inhibit SARS-CoV-2 entry into host cells, antivirals to block SARS-CoV-2 replication or factors that could boost the antiviral response, monoclonal antibodies that target proinflammatory cytokines that drive the hyperinflammatory response, and therapeutics that can improve the function of the lungs.
"Our hope is that the information contained in our review will help professionals in COVID-19 research develop new tools and agents to better treat those at high risk of severe COVID-19," Puig explained.
Do you have a unique perspective on your research related to immunology or infectious disease? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net