August 13, 2021 -- Researchers have demonstrated that heteroduplex oligonucleotide drugs conjugated with cholesterol efficiently cross the blood-brain barrier and can be used to treat central nervous system (CNS) diseases. The findings were published in Nature Biotechnology on August 12.
Antisense oligonucleotide (ASO) therapeutics are molecules that contain 18 to 30 base pairs that modify expression of a target mRNA, with the goal of suppressing harmful proteins or noncoding RNAs. These base pairs are ordered in reverse (antisense) and prevent the production of pathogenic proteins through binding to the "sense" strand of mRNA targets.
In general, only small hydrophobic molecules with a molecular weight of less than 450 dalton can penetrate the blood-brain barrier and the blood-cerebrospinal fluid barrier. ASOs are highly polar and thus generally do not enter the brain intact after system administration.
Researchers from Tokyo Medical and Dental University, Takeda Pharmaceutical, and Ionis Pharmaceuticals have developed DNA/RNA heteroduplex oligonucleotides (HDOs) conjugated to cholesterol (Chol-HDO) or alpha-tocopherol (Toc-HDO) as a drug delivery platform. They hope the technology will overcome the limited efficacy of ASOs targeting the CNS without requiring intrathecal administration.
The scientists synthesized and evaluated 14 lipophilic conjugates, including nine natural fatty acids and sterols, for gene knockout in the mouse CNS. The single-stranded ASO and the lipid-conjugated HDOs were injected intravenously at a dose of 50 mg per kg. Levels of target gene expression -- metastasis-associated lung adenocarcinoma transcript 1 (Malat1) RNA -- were measured in the cerebral cortex by quantitative polymerase chain reaction (PCR) with reverse transcription (qRT-PCR).
The team found that HDOs distribute throughout the brain, spinal cord, and peripheral tissues to suppress the expression of target genes by up to 90% in the CNS, whereas single-stranded ASOs conjugated to cholesterol have limited activity.
"We found that cholesterol conjugated HDO (Chol-HDO), unlike cholesterol-ASO, efficiently reached the CNS following subcutaneous or intravenous administration in experimental animals," said first author Tetsuya Nagata, PhD, an associate professor at Tokyo Medical and Dental University, in a statement. "The Chol-HDO platform showed significant dose-dependent target gene reductions with prolonged action in all CNS regions and cell types."
Furthermore, the longer-chain fatty acid conjugates -- lauroyl (C12), lauroyl (C12OH), stearoyl (C18), and alpha-tocopherol -- showed modest knockdown of Malat1 in the CNS, but the cholesterol conjugate was the most effective, reducing Malat1 levels by approximately 60% with a single intravenous injection. The authors suggested that cholesterol and tocopherol, which have a cyclic structural framework, were more effective than straight-chain fatty acids.
They also investigated the pharmacokinetics of multiple injections, as well as subcutaneous dosing. In vivo, repeated injections of Chol-HDO reduced Malat1 in the CNS by around 90%, with an approximate 50% reduction observed with the Toc-HDO. This RNA reduction by Chol-HDO was sustained throughout the brain for more than two months. The results in mice were also confirmed in rat models.
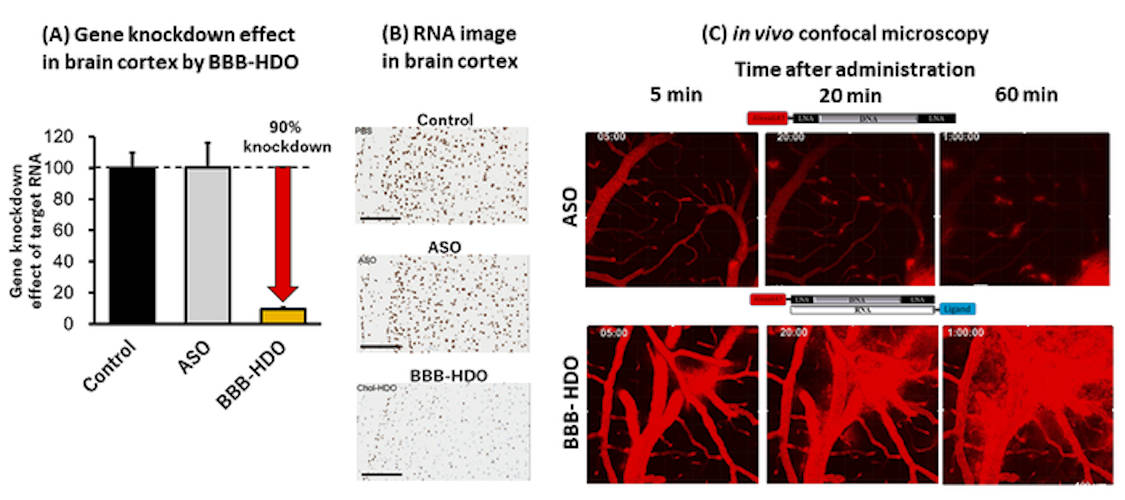
The compounds were generally well tolerated, even though higher accumulation of ASO was observed in peripheral organs, such as the liver and kidney, relative to the CNS. The authors noted that, in particular, the biodistribution of Chol-HDO is substantially greater to the peripheral tissues than to the brain.
"Systemic doses being higher, adverse effects such as mild decrease in platelets were expected," said Nagata. "However, divided or subcutaneous dosing can rescue these. We may also strategize by initiating treatment with intrathecal dosing to rapidly achieve therapeutic concentrations, followed by intravenous or subcutaneous maintenance as needed."
"Our innovative therapeutic platform for blood-to-brain delivery of ASOs may revolutionize management of neurodegenerative diseases," said senior author Dr. Takanori Yokota, PhD, a professor at Tokyo Medical and Dental University. "Future research will help define the specific molecular pathways, thus optimizing delivery of ASO pharmacotherapy to the CNS."
Do you have a unique perspective on your research related to neuroscience or genetic medicine? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net