July 26, 2021 -- Optogenetic therapy bypasses traditional methods of repairing defective photoreceptors in the eye by targeting other retinal cells. Recently, three examples of optogenetic therapeutics under development highlight many of the challenges this type of gene therapy faces for the treatment of a devastating genetic eye disease.
Retinitis pigmentosa (RP) is an inherited genetic disease caused by a mutational defect in any one of more than 70 different genes responsible for the photoreceptors on the retina. The disease progressively destroys the photoreceptor cells in the retina, beginning with the low-light sensing rods and eventually spreading to the color-sensitive cones. It affects about 200,000 people in the U.S. and 2 million people worldwide. Symptoms usually begin with poor night vision and typically lead to more serious vision problems such as the following:
- Sensitivity to bright lights
- Poor peripheral vision
- Tunnel vision
- Loss of depth perception
- Blindness
Optogenetic therapy targets retinal cells along the vision circuit pathway by modifying cells to express opsins, which are proteins that play a role in converting detected light into electrochemical signals. For instance, expression of opsin proteins on the surface of these cells transforms them into functional photoreceptor cells, allowing them to detect visible light directly, then passing those signals on to the brain's visual center.
Limited efficacy
Paris-based GenSight Biologics' optogenetic therapy, GS030, uses gene therapy in combination with an electronic prosthetic device to treat RP. The gene therapy uses a modified serotype 2 adeno-associated viral (AAV) vector to target retinal ganglion cells in the fovea. Ganglion cells handle the retina's output signaling and carry visual information to the brain.
The engineered ganglion cells are transduced to express a recombinant fusion protein containing ChrimsonR (an artificial opsin protein derived from a green algae channelrhodopsin) on their cell surfaces. The expressed fusion protein alters the cells to create signals in response to light, effectively transforming them into new photoreceptor cells.
The outcome is somewhat limited, however. The ChrimsonR protein is only sensitive to a narrow range of yellow light, with peak sensitivity at a wavelength of 590 nm, so the gene therapy treatment alone cannot produce a meaningful result in ambient light.
To address this concern, GeneSight's therapy includes a second component -- a set of electronic goggles designed to transform visible light into a format more easily detected by the transformed ganglia. The device uses a camera to measure changes in light intensity, then projects those changes into the treated retina using a 595-nm light source.
A recent case study published in Nature Medicine describes a 58-year-old RP patient participating in a phase I/IIA clinical study of GenSight's GS030 treatment. Beginning with limited light perception, after treatment and training with the googles, researchers provided evidence of improvements in vision associated with increased object-related extracranial multichannel electroencephalography measurements in the visual cortex of the brain.
Seven patients have already been treated with GenSight's therapy in three dose-escalation cohorts. The subject of the paper is a member of the lowest dose cohort; the other six patients have not yet been evaluated because of COVID-19. After the RP clinical trials have concluded, GenSight plans to investigate GS030 as a potential therapeutic to treat geographic atrophy in advanced age-related macular degeneration.
Meanwhile, Bedford, TX-based Nanoscope Therapeutics is using a proprietary AAV2 vector to transduce ON-bipolar cells in the retina to express a gene encoding a multicharacteristic opsin (MCO) on their surfaces. Bipolar cells normally serve as intermediates in the retina, passing signals from rod and cone photoreceptor cells on to the ganglion cells. Unlike other approaches, Nanoscope's MCO gene product is responsive to a broad range of visible wavelengths, making it a practical solution in natural ambient light, which eliminates the need for goggles.
Nanoscope has evaluated the MCO treatment in a phase I/IIA study, which included 11 patients with advanced RP. All patients had little or no light perception in the treated eye. Initially, three patients were given a low dose per eye, and three additional patients were given a high dose. Once the safety of the higher dose was established, five more patients were treated with the higher dose.
Patients reported long-lasting improvements in outdoor light sensitivity and daily activities, according to clinical investigators. One year post-treatment, six out of seven of the high-dose patients showed a visual acuity improvement of 15 letters or more. (The eighth member of the cohort was not examined after 31 weeks due to COVID-19.)
Nanoscope Therapeutics is planning a late-stage phase IIB trial of the treatment this summer. Concurrently, the company has launched phase I clinical trials of the treatment for Stargardt disease and is planning clinical trials for the treatment of cone-rod dystrophy and Usher syndrome.
Complex neural processing
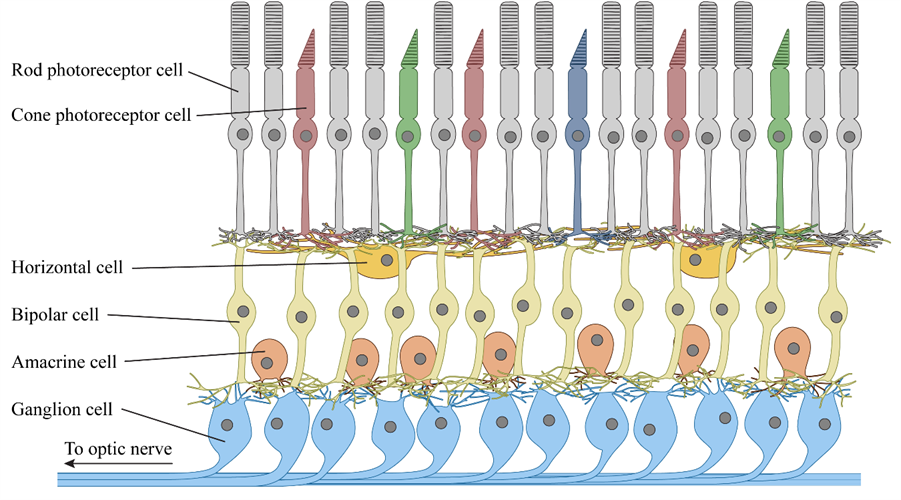
Retinal ganglion and bipolar cells do more than simply pass signals from rod and cone photoreceptor cells on to the brain. The human retina comprises multiple cell types arranged in a complex neural network, which performs a series of operations on the visual data it receives. With about 100 times as many photoreceptor cells as ganglion cells in a typical human retina, each ganglion cell receives input from anywhere between a handful and several thousand photoreceptors.
Quite a bit of sensory data processing and encoding occurs within the retina itself to translate all that visual information into a language and format the brain can quicky understand and interpret. Modeling that processing is another major challenge of optogenetic therapies.
New York-based Bionic Sight believes it can overcome that problem by faithfully emulating the data processing that normally occurs in a healthy retina. Its BS01 gene therapy for RP uses an AAV2 vector to transduce retinal ganglion cell to express ChronosFP, a modified channelrhodopsin, which appears to have greater sensitivity and responsiveness than earlier optogenetic proteins.
Bionic Sight's most important innovation is its advanced goggle apparatus. The neuroprosthetic device uses a sophisticated model to process images taken by a camera and encode them into a series of pulses, simulating the processing and encoding steps that normally take place in a human retina before the visual data are sent to the brain. The encoded pulses (now in a format the brain can readily understand) are then projected by the goggles onto the retina, where the transduced ganglion cells convert them to signals that are passed on to the brain.
Bionic Sight showed measurable effects of the BS01 therapy in a phase I/II clinical trial. Patients were treated with two different BS01 doses, then subjected to light and motion tests at three months and six months post-treatment. The goggle device was introduced at the six-month test. The two patients given the lowest dose showed a greater than 20-fold increase in light sensitivity, while the two patients given a higher dose showed a greater than 100-fold increase. At least one patient reported that he can recognize shapes using the goggles and has also reported improved vision even without the goggles.
Bionic Sight partnered with Florida-based Applied Genetic Technologies Corporation in 2017 to develop its optogenetic gene therapy vector, which was manufactured at the Belfer Gene Therapy Core Facility at Weill Cornell Medicine. Research partners for clinical trials include Pharmaceutical Research Network (an ophthalmic contract research organization) and Ophthalmic Consultants of Long Island.
Blake Middleton is the editor of Cell and Gene Therapy Business Outlook, part of Science and Medicine Group.
Disclosure: Cell and Gene Therapy Business Outlook is a sister publication of ScienceBoard.net.
Do you have a unique perspective on your research related to gene therapy? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net