April 28, 2023 -- Researchers have created a lentiviral gene therapy vector capable of targeting muscle cells to treat the rare disease Duchenne muscular dystrophy (DMD) in mice.
A DMD gene therapy based on an adeno-associated virus (AAV) delivery platform, Sarepta Therapeutics' SRP-9001, is currently under review at the U.S. Food and Drug Administration (FDA) and rival candidates are in clinical development. However, there is scope to improve on the current programs -- SRP-9001 failed to improve functional motor ability scores in a phase 2 clinical trial -- and new delivery vehicles may be key.
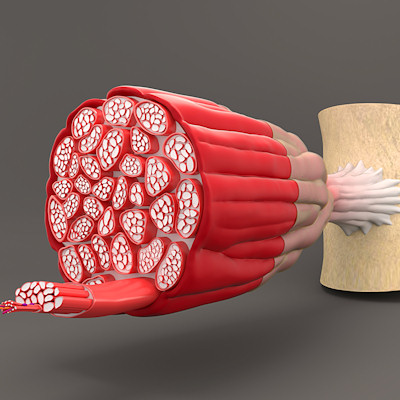
Writing in Cell, scientists at Cincinnati Children's Hospital Medical Center recently shared details of their attempt to develop a new way to get genetic material to muscle cells to correct the root cause of DMD. Patients with the rare disease have alterations in a protein that helps keep muscle cells intact.
Rather than use an AAV vector, the researchers modified a lentivirus to target muscle cells. The targeting was enabled by two proteins, known as fusogens, that coordinate membrane fusion and mediate entry of stem cells into mature muscle cells. By engineering the fusogens Myomaker and Myomerger on the membrane of enveloped viruses, the scientists created vectors that specifically fuse with muscle cells.
"We envision that this concept, transferring a naturally occurring process within muscle to membrane vehicles, could revolutionize delivery of therapeutic material to skeletal muscle to improve genetic conditions such as muscular dystrophy and conditions associated with muscle loss and weakness," Doug Millay, PhD, a scientist at Cincinnati Children's, said in a statement.
Millay and his collaborators were unsure going into the project if Myomaker and Myomerger, proteins that have different structures than classical fusogens, would work on viruses. The study suggests that the approach can enable the targeted delivery of a gene that encodes micro-dystrophin, a smaller version of the protein at the heart of DMD.
After administering the lentivirus to mice, the researchers detected micro-dystrophin in 5% to 25% of the muscle cells in limb muscles and 77% to 90% of muscle cells in the diaphragm. The level of delivery to the diaphragm was high enough to drive reduced central nucleation, a marker for muscle degeneration, and fibrosis in dystrophic mice.
Muscle-trophic AAV vectors already exist but the researchers see benefits to using lentiviruses, noting that the delivery vehicles have a larger packaging capacity. AAVs can only carry genetic payloads of up to 4.8 kilobytes, compared to 8 kilobytes for lentiviruses, which limits the diseases that they can treat. Even so, lentiviruses lack the capacity to carry the full-length dystrophin gene.
Copyright © 2023 scienceboard.net