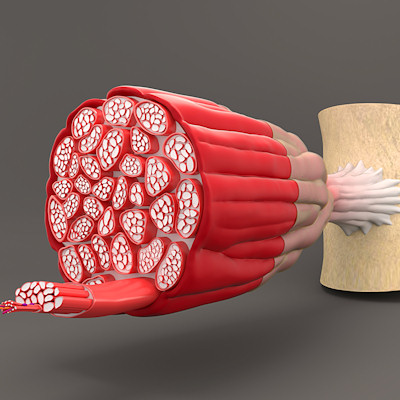
September 13, 2022 -- Researchers at Johns Hopkins Medicine have discovered that blocking an ion channel increases muscle function and survival in mice with severe Duchenne muscular dystrophy (DMD), a genetic disorder that progressively weakens skeletal and heart muscles over time.
Their study, published September 13 in the Journal of Clinical Investigation - Insight, found that a drug blocking the ion channel -- called TRPC6 -- in genetically modified mice with severe DMD doubled their survival and improved their skeletal and cardiac muscle function, while reducing bone deformities associated with weak muscles.
Gene mutations causing loss of the protein dystrophin result in the severe muscle disease, which primarily affects boys who show symptoms between the ages of 2 and 4. According to the Muscular Dystrophy Association, thanks to advances in cardiac and respiratory care, survival into the early 30s is becoming more common.
"We believe this may be one of the first studies to prolong survival in this very severe model of Duchenne with a compound that can be administered orally. While the molecule does not restore dystrophin, it blocks abnormal behavior of another protein that is caused by the lack of dystrophin," senior author Dr. David Kass, professor of cardiology at the Johns Hopkins University School of Medicine, said in a statement. "This is not a cure for Duchenne, but if human trials replicate these findings, it could mean patients may survive into their late 40s and 50s with the possibility for an improved quality of life."
The researchers treated male and female DMD mice with a TRPC6 inhibitor drug (BI 749327, developed by Boehringer-Ingelheim Pharmaceuticals) or with a placebo. According to the authors, the same ion-inhibiting drug given to mice in their study is in clinical trials to treat people with kidney disease, so clinical studies in patients with Duchenne could start soon since the drug has already been proven safe in humans.
Copyright © 2022 scienceboard.net