July 23, 2020 -- New tools using surrogate viruses may be useful for rapid testing to determine whether antibodies effectively neutralize SARS-CoV-2. The viral vector-based platform approach was published in the Journal of Experimental Medicine on July 21.
Coronaviruses like SARS-CoV-2 elicit neutralizing antibodies that provide protection against reinfection. However, protection is incomplete and diminishes over time. The evidence for SARS-CoV-2 thus far indicates that infection causes relatively low to undetectable levels of plasma-neutralizing antibodies from convalescent patients.
Human monoclonal antibodies may be an alternative to convalescent plasma for providing antibody-based protection against SARS-CoV-2. Neutralizing antibodies could be a potent therapy or prophylaxis against COVID-19 and are of interest for vaccine and therapy development.
"Whether elicited by natural infection or vaccination, or administered as convalescent plasma or in recombinant form, neutralizing antibodies will likely be crucial for curtailing the global burden of COVID-19 disease," said Paul Bieniasz, PhD, a professor at Rockefeller University and investigator at the Howard Hughes Medical Institute, in a statement. "For this reason, the availability of rapid, convenient, and accurate assays that measure neutralizing antibody activity is crucial for evaluating naturally acquired or artificially induced immunity against SARS-CoV-2."
Traditional plaque reduction neutralization tests are laborious, require biosafety level 3 (BSL3) facilities, and cannot be conducted in a high-throughput manner.
As an alternative, pseudotype virus assays may provide a high-throughput testing option that can be performed at BSL2 facilities. However, the ability of these assays to predict plasma neutralization activity against SARS-CoV-2 or identify potent human monoclonal antibodies has not been thoroughly evaluated.
Researchers from Rockefeller University evaluated assays based on pseudotyped and chimeric viruses to measure neutralizing activity of convalescent plasma and to identify potent neutralizing human monoclonal antibodies against SARS-CoV-2. They used a panel of convalescent plasma and human receptor-binding domain-specific monoclonal antibodies to demonstrate that the assays were effective and can be used to estimate SARS-CoV-2 immunity.
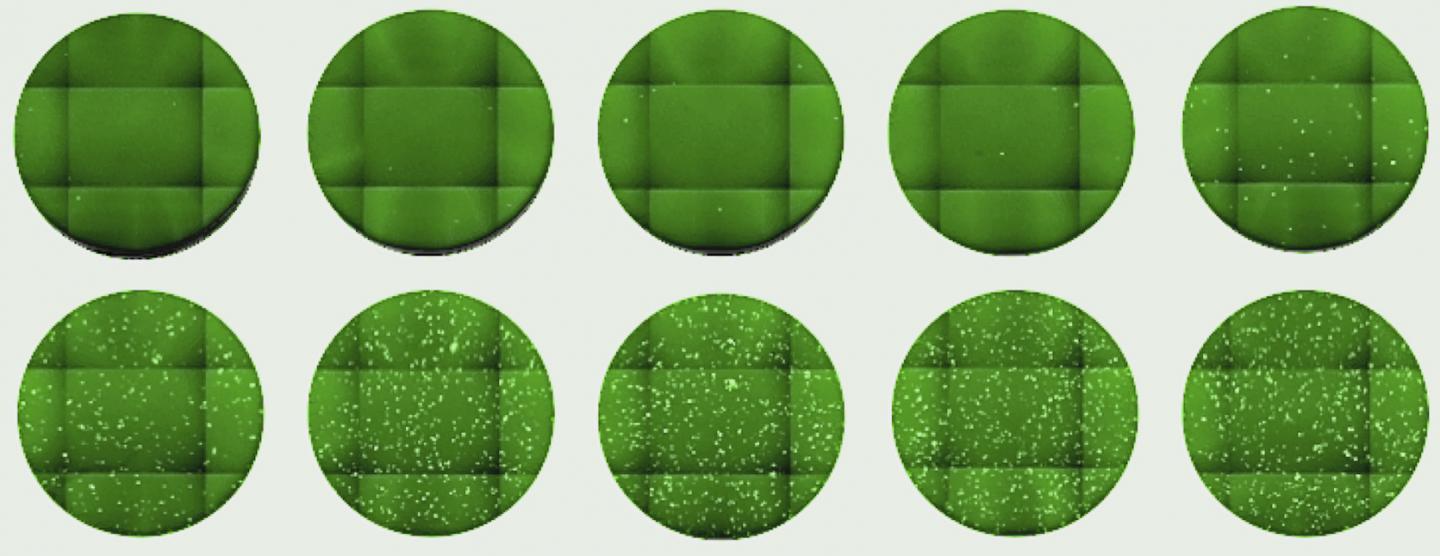
The first assay utilized HIV-1-based SARS-CoV-2 pseudotyped virons. This plasmid-based system was constructed using plasmids encoding codon-optimized SARS-CoV-2 proteins. The HIV-1 pseudotype infection assays were formatted in 96-well plates with a dynamic range to accurately determine plasma-neutralizing activity as well as potency of monoclonal antibodies or other inhibitors of SARS-CoV-2 spike-dependent viral entry.
The second platform for evaluating viral envelop or spike protein function was based on vesicular stomatitis virus (VSV). This system contained a dual reporter that could be monitored by flow cytometry or NanoLuc luciferase assay. The VSV-NanoLuc SARS-CoV-2 pseudotype infection assay also had a dynamic range of three to four orders of magnitude within 96-well plates. The relationship between input virus dose and signal was linear in this assay.
The HIV-1 and VSV-based pseudotyped virus assays were somewhat less sensitive to neutralization than authentic SARS-CoV-2. This finding may be because they are single-cycle assays or because the pseudotyped virons have lower spike density than SARS-CoV-2. Each of the assays displays subtle differences in sensitivity.
However, "each of the surrogate virus-based assays generated quantitative measurements of neutralizing activity that correlated well with neutralization measured using authentic SARS-CoV-2," said study co-director Theodora Hatziioannou, PhD, research associate professor at Rockefeller. "In just a few weeks, we have already used these assays to determine the neutralizing potencies of hundreds of plasma samples and monoclonal antibodies in a biosafety level 2 laboratory."
Importantly, automation and additional miniaturization of these assays could increase throughput to accelerate vaccine candidate development pipelines. The authors suggested that these pseudotyping approaches to detect neutralizing potencies of plasma samples and monoclonal antibodies in BSL2 laboratories could be an asset for determining if patients are susceptible to reinfection by SARS-CoV-2 and assessing the effectiveness of experimental vaccines.
Do you have a unique perspective on your research related to virology or infectious disease research? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net