August 19, 2020 -- New research demonstrates that the SARS-CoV-2 spike protein may be more flexible than previously thought, with hinges similar to leg joints to seek receptors on a host, according to a new article published in Science on August 18. Understanding molecular dynamics of how the spike protein functions could have implications in therapeutic and vaccine design.
The SARS-CoV-2 spike protein is a trimeric class I viral fusion protein with a club-like shape that is approximately 20 nm in length. The ectodomain is connected to the viral membrane by a slender stalk. The three receptor-binding domains of the ectodomain change conformation and are protected by N-terminal domains.
In the open conformation of the virus, one receptor-binding domain is exposed. Previous studies have shown that about two-thirds of the spike protein is covered with N-glycans that are thought to shield it against antibodies.
These observations have been studied extensively with recombinant protein in vitro, yet it remains unknown if the distribution and conformational states and the glycosylation patterns are representative of the native state generated during viral assembly.
"The upper spherical part of the spike has a structure that is well reproduced by recombinant proteins used for vaccine development," explained Martin Beck, PhD, group leader at the European Molecular Biology Laboratory (EMBL) and a director of the Max Planck Institute (MPI) of Biophysics, in a statement. "However, our findings about the stalk, which fixes the globular part of the spike protein to the virus surface, were new."
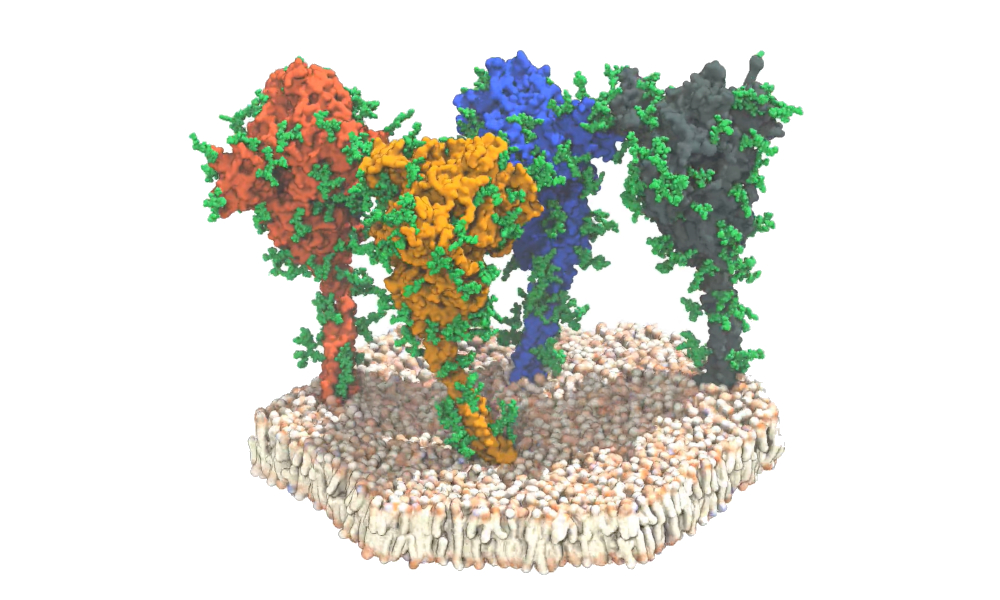
To evaluate viral spike proteins, a team of German researchers combined cryoelectron tomography, subtomogram averaging, and molecular dynamics simulations to analyze the structure of the spike protein in its natural environment.
Using EMBL's cyroelectron microscopy facility, 266 cryotomograms (images) covering more than 1,000 viruses -- each with an average of 40 spike proteins -- were generated. The researchers observed that around half of the spike ectodomain was in the fully closed conformation while a smaller fraction had one receptor-binding domain exposed.
In contrast, they found that the stalk connecting the spike ectodomain to the viral membrane was highly dynamic. The stalk contains an 11-residue leucine repeat sequence and adopts an unusual right-handed coil, which the researchers call the "upper leg" or "hip." The images reveal the presence of flexible hinges in the stalk that allow the ectodomain to tilt up to 90° at distances of 5 nm to 35 nm from the membrane. The section below this coil and the flexible hinge is the section termed the "lower leg" or "knee."
Molecular dynamics simulations confirmed that the stalk exhibits pronounced hinging motions at the junctions between the upper leg and the lower leg and the transmembrane domain ("ankle"). The hip joint flexed the least, followed by the ankle and knee. The movement may facilitate motions that interfere with antibody access to the stalk.
"The stalk was expected to be quite rigid," added team member, Gerhard Hummer, PhD, from MPI of Biophysics and the Institute of Biophysics at Goethe University Frankfurt. "But in our computer models and in the actual images, we discovered that the stalks are extremely flexible."
The researchers found that glycosylation sites are very bulky in the tomographic maps, suggesting that sugars may be more extensive during viral assembly as compared to recombinant ectodomain. They also observed the number of glycans on the spike proteins had more pronounced branching than previously thought. The team found additional glycosylation at the hinges of the stalk domain and on the very tips of the N-terminal domains. These sugar-like molecules act as a protective coat that helps hide spikes from neutralizing antibodies.
Overall, the team suggested that the flexible spike protein acts like a balloon on a string with the ability to move on the surface of the virus and search for the receptor for docking to target cells.
Do you have a unique perspective on your research related to electron microscopy or virology? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net