November 10, 2020 -- Factor Bioscience has received its second U.S. patent in 2020 for its messenger RNA (mRNA) vectorization technology of transcription activator-like effector nucleases (TALENs).
U.S. patent No. 10,829,738 covers methods for producing gene-edited cells using mRNA encoding TALENs. This patent, and the other patent (No. 10,662,410) awarded to Factor by the U.S. Patent and Trademark Office, form the foundation of clinical-stage allogeneic chimeric antigen receptor (CAR) T-cell therapies, according to the company. Their methods are used to inactivate the endogenous T-cell receptor to prevent therapeutic T cells from causing graft-versus-host disease (GvHD) and to perform other key cell engineering steps, Factor said.
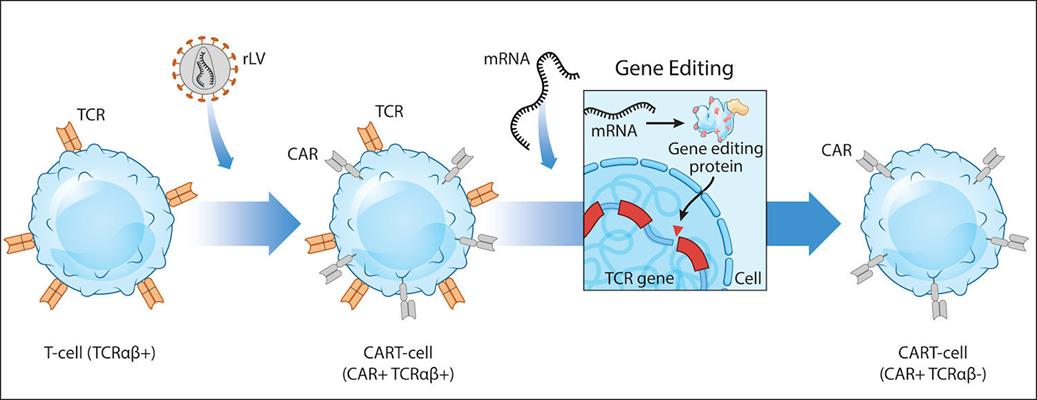
In addition, these patented methods are also used in applications such as generation of allogeneic stem cell-derived therapies. In these treatments, mRNA encoding TALENs are used to inactivate components of the human leukocyte antigen (HLA) complex to render the cells immuno-nonreactive and to insert donor sequences into defined genomic loci to enable controlled expression of exogenous genes, according to the company.
Other patents are currently pending in the U.S. and other countries, Factor said.
Copyright © 2020 scienceboard.net










