September 22, 2020 -- Researchers have developed a new 2D lung organoid in vitro model as a physiologically relevant platform to aid drug development and explore the pathogenesis of SARS-CoV-2 infection. Details were published online in Cell Stem Cell on September 18.
Distal lung alveoli that express the cell surface receptor for SARS-CoV-2, angiotensin-converting enzyme 2 (ACE2), allow for infection and can lead to respiratory distress syndrome associated with COVID-19 pneumonia. Severe infection targets the epithelium of the lung, particularly the progenitor stem cells in the region, alveolar epithelial type 2 cells (type II pneumocytes). These cells are conventionally difficult to access in vivo and difficult to maintain in vitro.
To date, SARS-CoV-2 infection modeling has been predominantly performed using human airway (nonalveolar) cells in air-liquid interface cultures, nonhuman cell lines that naturally express the ACE2 viral receptor (e.g., the Vero E6 cell line), or transformed human cell lines with or without forced over-expression of ACE2.
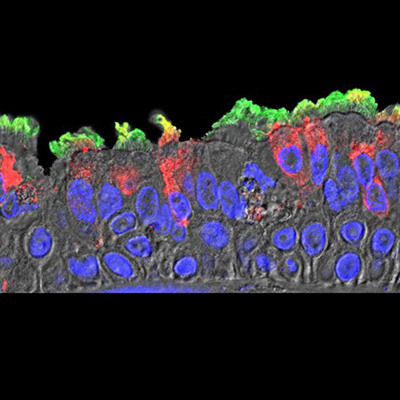
Now, a team of infectious disease, pulmonary, and regenerative medicine researchers at the Boston University School of Medicine (BUSM) have developed an in vitro human model that simulates the initial apical infection of alveolar epithelium with SARS-CoV-2, using induced pluripotent stem cell-derived alveolar epithelial type 2 cells that have been adapted to air-liquid interface culture.
The model was adapted from a previously established research model used to study the effects of smoking cigarettes. In the current study, droplets of live virus were added on top of lung cells infecting them from the air as would happen inside the lungs when air containing the virus is breathed into the body.
"This adaptation of human stem cell-derived pneumocytes to air, known as an 'air-liquid interface' cell culture, was a key advance that allowed us to simulate how SARS-CoV-2 enters cells deep in the lungs of the most severely affected patients," said co-senior author Dr. Andrew Wilson, associate professor of medicine at BUSM, in a statement. "Type 2 pneumocytes are also infected and injured in patients with COVID-19, making this a clinically meaningful system to understand how the disease injures patient lungs."
Using the in vitro model, the researchers implicate the stem cells as inflammatory signaling centers that respond to SARS-CoV-2 infection within 24 hours with a predominantly nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) signaling response.
"The infected lung cells pour out inflammatory proteins," explained author Dr. Darrell Kotton, the David C. Seldin Professor of Medicine at BUSM and director of the BU/Boston Medical Center for Regenerative Medicine. "In the body of an infected person, those proteins drive up levels of inflammation in the lungs."
Gene set enrichment of RNA sequencing samples identified a shift to an inflammatory phenotype associated with the secretion of cytokines encoded by NF-kB target genes on the first and fourth days post-infection. The data suggest that SARS-CoV-2 infection of the stem cells elicits a delayed, modest interferon response.
"Our data confirms that SARS-CoV-2 blocks cells from activating one of the antiviral branches of the immune system early on after infection has set in," added Kotton. "The signal the cells would typically send out, a tiny protein called interferon that they exude under threat of disease, are instead delayed for several days, giving SARS-CoV-2 plenty of time to spread and kill cells, triggering a buildup of dead cell debris and other inflammation."
Moreover, the researchers found that alveolar epithelial type 2 cell-specific genes were among some of the most downregulated genes and led to a progressive loss of mature alveolar epithelial programs. Paired with increases in cell death-related genes, which were upregulated, the results suggest that SARS-CoV-2 infection of the alveolar stem cells leads to a cell-intrinsic shift away from a normal development program toward an inflammatory program.
"These cells are an amazing platform to study SARS-CoV-2 infection," noted Elke Mühlberger, PhD, associate professor of microbiology at BUSM and a researcher at BU's National Emerging Infectious Diseases Laboratories. "They likely reflect what is going on in the lung cells of COVID-19 patients. If you look at the damage SARS-CoV-2 inflicts on these cells, you definitely don't want to get the disease."
Do you have a unique perspective on your research related to virology or infectious disease research? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net