August 9, 2022 -- Researchers at the University of Vienna have come up with an unconventional way toward overcoming the traditional concentration limitation for determining the structure and dynamics of proteins using nuclear magnetic resonance (NMR) spectroscopy.
While NMR enables complete structural characterization of biomolecules with atomistic resolution in their native solution environment, due to the intrinsically low sensitivity of the method the target molecule's concentration must be much higher than found in the body's cells.
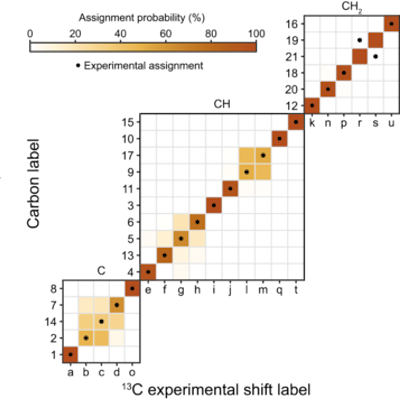
However, in an August 5 article in the journal Science Advances, researchers describe how hyperpolarization -- more precisely by dissolution dynamic nuclear polarization -- can be used to achieve a 1,000-fold signal amplification in NMR measurements.
"Spectroscopy bears some similarities with an electric guitar: If the amplifier is too weak, you will hear very little if you do not hit the strings strongly," Dennis Kurzbach, PhD, at the Institute of Biological Chemistry, University of Vienna, said in a statement. "Meaning that you need a lot of material to see an NMR signal. With the new hyperpolarization amplifier, you can now see something even at low concentration."
According to the authors, their new method can help to better understand the process of cell proliferation to tumor growth, helping to elucidate basic mechanisms for cancer development.
Copyright © 2022 scienceboard.net