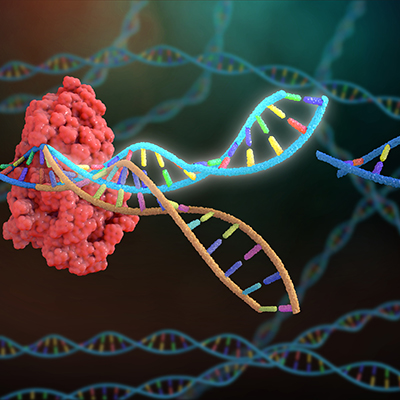
July 21, 2022 -- An integrated experimental and computational method could make site-specific recombination more efficient and predictable. A research team used the model to predict and control simultaneous production of multiple proteins within a cell, pointing to applications in regenerative medicine and cell therapy.
Researchers at the University of Minnesota, Twin Cities developed the experimental model to calculate the rate of site-specific recombination and then used it to train a machine-learning algorithm. Their study was published July 20 in Nature Communications.
"To our knowledge, this is the first example of using a model to predict how modifying a DNA sequence can control the rate of site-specific recombination," said Casim Sarkar, PhD, associate professor in the University of Minnesota, Twin Cities Department of Biomedical Engineering and senior author on the paper, in a statement. "By applying engineering principles to this problem, we can dial in the rate at which DNA editing happens and use this form of control to tailor therapeutic cellular responses."
Site-specific recombination technology uses enzymes, known as recombinases, that can recognize two DNA sites and recombine them in a new configuration. The process enables the gene insertions, deletions, and inversions that are needed to create many cell therapies (for example by modifying the genes to empower a patient's own cells to attack cancer).
The timing of gene expression affects the outcome. Recognizing that, Sarkar worked with colleagues to make the process more efficient and predictable. The researchers developed a quantitative polymerase chain reaction (qPCR)-based assay to quickly capture initial site-specific recombination rates in vitro.
Building on the assay, Sarkar and his collaborators developed a data-driven method to predict the relative reaction rate as a function of nucleotide sequence. The approach enabled the programming of the reaction rate over four orders of magnitude through nucleotide substitutions.
Making multiple substitutions enabled notable improvements. While each substitution had a modest effect on its own, the researchers were able to create sequences that conferred a 10-fold increase in the initial reaction rate over the wild type by combining multiple substitutions. The researchers believe the sequences should be useful in improving efficiency in a range of synthetic biology and cell engineering applications.
The team also sees opportunities to expand the data-driven approach to other recombinases and DNA or RNA modifying enzymes, provided an appropriate selection system can be developed. Ultimately, the researchers see the work further broadening "the genetic editing toolbox" and improving "the ability to design tunable artificial circuits."
Copyright © 2022 scienceboard.net