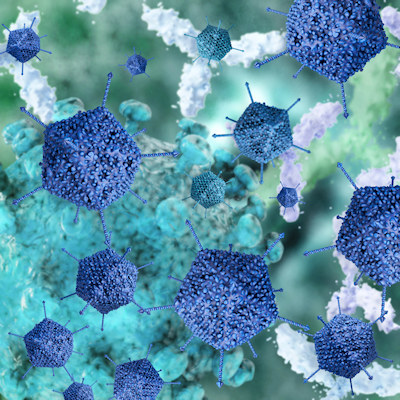
September 10, 2021 -- Scientists have engineered a new family of adeno-associated viruses (AAVs) to deliver therapeutic genes to muscle tissue at 100 to 250 times lower doses than previous AAV vectors, potentially reducing the risk of liver damage and other serious side effects, according to a new study published in Cell on September 9.
Using AAV vectors to deliver a gene therapy has shown promise in clinical trials for a subset of muscular dystrophies, such as Duchenne muscular dystrophy (DMD), and X-linked myotubular myopathy, but the technology is still far from perfect. One challenge is that AAVs tend to target liver tissue rather than the intended muscle tissue.
To reach the muscles throughout the body, high doses of the virus are needed, but the AAVs used in these trials often are stored in the liver more than in the muscle. This has led to high levels of the virus in the liver, severe adverse side effects, and even death in some trial participants.
"Scientists have been doing preclinical work for in vivo gene therapy for the last 15 years and have made tremendous progress," said first author Sharif Tabebordbar, PhD, a research scientist at the Broad Institute of MIT and Harvard, in a statement. "We know that if you get enough of the drug into the target tissue, it's going to be efficacious. It's all about delivering a safe dose of the virus."
Engineering AAV capsids
Tabebordbar and his colleagues at the Broad Institute and Harvard University have developed a strategy to target the muscles more efficiently with the gene-carrying virus.
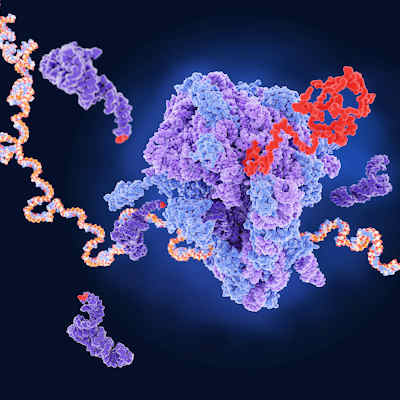
The technique they developed, called directed evolution of AAV capsids leveraging in vivo expression of transgene RNA (DELIVER), generates millions of different AAV capsids by adding random strings of amino acids to the portion of the AAV capsid that is exposed on the surface of the virus and binds to the targeted cells. In preclinical studies, these modified AAV capsids are injected into mice and nonhuman primates to identify the ones that successfully delivered their genetic cargo to the intended muscle tissue.
"There have been many capsid engineering studies, which we've learned a lot from, but what we've done here is very comprehensive," said Tabebordbar. "We've evolved a family of capsids, found the mechanism by which it delivers genes, showed that this mechanism is conserved between species, and showed that we can provide a therapeutic benefit in animal models with an extremely low dose of the virus. Now, we're extremely excited about how this can be used to enable effective drug development for patients."
The DELIVER process revealed a family of capsid variants -- MyoAAV -- with a unique surface structure that specifically targets muscle cells. In testing, MyoAAV vectors demonstrated transduction potency (the ability to deliver genetic material into cells) across mouse, nonhuman primate, and human muscle cells.
Putting engineered AAVs to the test
Two promising members of the MyoAAV family, MyoAAV 1A and MyoAAV 2A, showed interesting, species-specific characteristics as delivery vectors for gene editing and gene therapies. Specifically, MyoAAV 2A proved highly potent at delivering genetic material into the muscles of mice. The top primate capsid variants also proved highly potent in mice, suggesting they are good candidates for therapeutic development.
In a test of MyoAAV 2A's gene-delivery abilities, the researchers compared it to AAV9, a capsid currently used in gene replacement trials for DMD. They injected MyoAAV 2A and AAV9 equipped with a microdystrophin transgene, which has been shown to improve outcomes in dystrophic mice, into a DBA/2J-mdx mouse model of DMD using a low dose (2E+13 vg/kg) of each AAV. The MyoAAV 2A-injected animals demonstrated greater and more widespread expression of microdystrophin in multiple muscle groups compared to AAV9-injected animals.
One characteristic that all of the top-performing MyoAAV capsids had in common was that they contained a common arginine-glycine-aspartic acid (RGD) motif. The RGD motif is known as the minimal sequence in fibronectin that facilitates binding to its receptor. Additional analysis showed that these capsids are dependent on integrin heterodimers for transduction across species.
The results of the study represent the culmination of 10 years of work by Tabebordbar, for whom the quest for better gene therapies for genetic muscle disease is personal. When Tabebordbar was a teenager, his father was struck with a rare genetic muscle disease that caused him to rely on a wheelchair.
"I watched my dad get worse and worse each day. It was a huge challenge to do things together as a family -- genetic disease is a burden on not only patients but families," said Tabebordbar. "I thought, 'This is very unfair to patients, and there's got to be a way to fix this.' That's been my motivation during the 10 years that I've been working in the field of gene therapy."
Do you have a unique perspective on your research related to gene therapy? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net