July 27, 2021 -- Researchers have identified a way to restrict regulatory T-cell (Treg) activity in tumor cells, which in turn can unleash other immune cells (conventional T cells) to attack tumors in cancer patients. The research is being published in the August 3 issue of the Proceedings of the National Academy of Sciences.
"A patient's immune system is more than able to detect and remove cancer cells, and immunotherapy has recently emerged as a novel therapy for many different types of cancers," explained lead author Nullin Divecha, PhD, a professor at the University of Southampton, in a statement. "However, cancer cells can generate a microenvironment within the tumor that stops the immune system from working thereby limiting the general use and success of immunotherapy."
Tregs play a key role in immune surveillance to prevent autoimmunity and can limit legitimate immune responses through suppression of effector T-cell (Teff) signaling and tumor cell killing.
Tregs express high levels of Treg lineage specification transcription factor Forkhead box P3 (FOXP3), which controls their function and identity. In tumors, Tregs represent up to 20% to 30% of CD4+ T cells.
"Tregs carry out an important function in the human body because without them, the immune system can run out of control and attack normal cells of the body," Divecha said. "However, in cancer patients, we need to give the Teff cells more freedom to carry out their job."
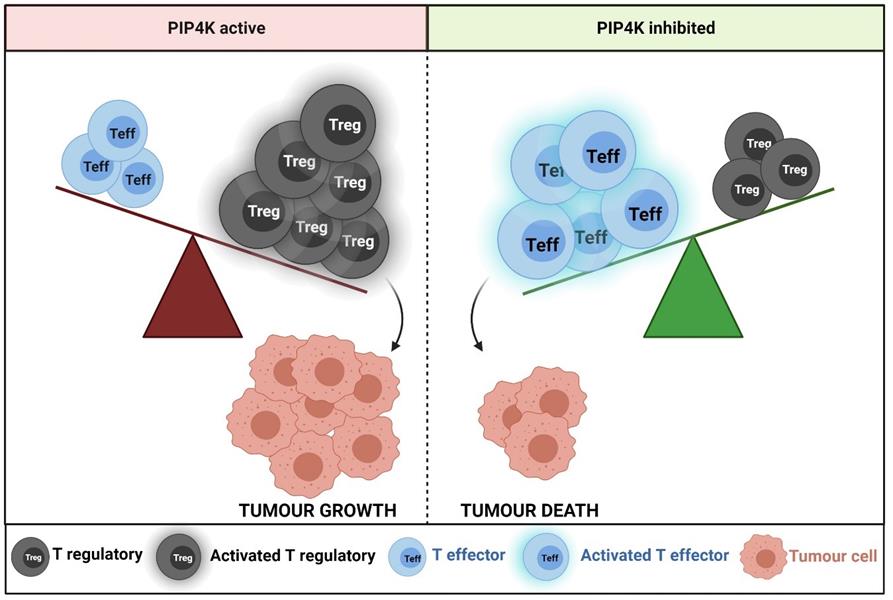
Treg depletion has the potential to boost antitumor immune responses. Pharmacological tuning of Treg activity -- without impacting conventional T-cell activity -- would be extremely beneficial. In the current study, researchers from the University of Southampton and the National Institute of Molecular Genetics in Milan found that a group of enzymes called phosphatidylinositol 5-phosphate 4-kinases (PIP4Ks) could be the answer to how Tregs can be restricted without affecting effector T cells.
There are three isoforms of PIP4Ks: 2A, 2B, and 2C. Loss of PIP4K2C in mice has been shown to lead to late-onset hyperinflammation and has been linked to susceptibility to autoimmune diseases.
Using healthy donors, the research team depleted Treg and PIP4K to determine the effect on immune responses in vitro. Loss of PIP4Ks from Tregs stopped them from growing and responding to immune signals, which in turn stopped them from blocking the growth and function of effector T cells. Depletion of PIP4K2B in Tregs changed the expression of 2,569 genes, while depletion of PIP4K2C resulted in only 524 changed genes. These sets of genes contained a high degree of overlap.
Importantly, when PIP4K was removed from effector T cells, activity was not lost.
"This was surprising because PIP4Ks are in both types of T cells in similar concentrations, but our study shows that they seem to have a more important function for Tregs than T effectors," said Alessandro Poli, PhD, a postdoc at the National Institute of Molecular Genetics and FIRC Institute of Molecular Oncology Foundation.
With genetic and pharmacological inhibition assays, the team demonstrated that PIP4K suppresses human Treg function in three ways. First, the enzymes control signaling through the phosphoinositide 3-kinase (PI3K), mammalian target of rapamycin complex 1 (mTORC1/S6), and mitogen-activated protein kinase (MAPK) pathways that control the cell cycle and mitosis. Second, they impact Treg proliferation and cause Treg cell death. In this case, conventional T-cell activation is left intact. Third, they control the expression of the master transcriptional regulator FOXP3 and, more broadly, reprogram Treg transcription profiles.
Inhibition of PIP4K as a potential therapeutic for patients requires the development of inhibitory molecules. The team tested an irreversible inhibitor of PIP4K2C depletion in human Tregs. The inhibitor increased immune responses in Tregs but had little effect in conventional T cells.
"Towards this end, we show that treatment with a drug-like inhibitor of PIP4K could enable the immune system to function more strongly and be better equipped to destroy tumor cells," Poli explained.
Do you have a unique perspective on your research related to cancer biology or immunology? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net