March 1, 2021 -- Researchers have described a new single-cell RNA-sequencing approach, leveraging genomic and mitochondrial DNA mutations, that has the potential to differentiate normal stem cells from cancer stem cells. The article detailing the approach was published in Nature Communications on March 1.
Acute myeloid leukemia is a good model to study cancer stem cells because 10%-20% of individuals over the age of 70 develop preleukemic mutations in hematopoietic stem cells. From here, the preleukemic stem cells can give rise to healthy blood and immune cells, but the acquisition of additional mutations could result in malignant expansion of aberrant leukemic stem cells.
These cancer stem cells can fuel long-term cancer growth with their ability to continuously regenerate. However, low division rates make them difficult to target therapeutically. Chemotherapy often misses the leukemic stem cells, leading to recurrent relapse. Therefore, the identification of therapeutic strategies that target leukemic stem cells, while sparing healthy hematopoietic stem cells, is urgently needed.
Researchers from the Centre for Genomic Regulation (CRG) and the European Molecular Biology Laboratory (EMBL) sought to develop an approach that could characterize the gene expression differences among healthy hematopoietic stem cells, preleukemia stem cells, and leukemia stem cells. Together, MutaSeq, amplifies nuclear mutations from complementary DNA (cDNA), and mitoClone, a computational tool that achieves high-confidence clonal assignments and de novo discovery of clones using mitochondrial marker mutations when available, allowed the team to use genomic and mitochondrial mutations to distinguish between healthy and cancerous cells.
They measured mutational status and gene expression in single cells simultaneously in order to distinguish cancer stem cells from both mature cancer cells (based on gene expression) and healthy stem cells (based on mutational status). Furthermore, they were able to distinguish preleukemia stem cells through the detection of clonal hematopoiesis of indeterminate potential (CHIP) mutations.
To explore the applications of MutaSeq in combination with mitoClone, the researchers generated data from four acute myeloid leukemia patients with heterogenous genotypes and phenotypes. The final data set consisted of 618-1430 cells per patient, of which 190-968 were CD34+ (an indicator of acute myeloid leukemia).
The researchers then clustered cells into clonal hierarchies (descendance relations between the different clones that reveal the order in which mutations have been acquired) based on nuclear genomic mutations, as well as mitochondrial mutations.
This technique revealed known preleukemia mutations, such as mutations in the SRSF2 and DNMT3A genes. However, relying on nuclear somatic mutations missed the identification of other mutations with lower expression. For these, the team used mitochondrial mutations to refine clonal hierarchies together with nuclear mutations. In two of the samples, they found that preleukemic, as well as subclonal leukemic mutations, were significantly associated with distinct sets of well-covered mitochondrial variants.
Across the four patients, the researchers identified clones carrying known preleukemic mutations (in SRSF2 and DNMT3A) and subclones carrying known leukemic mutations (CEBPA and NPM1).
On a de novo basis, the researchers used MutaSeq to identify clones associated with leukemic stem cells. They found a highly significant association of the serine and arginine rich splicing factor 2 (SRSF2) P95H mutation with the leukemic and preleukemic subclones and a DNA methyltransferase 3A (DNMT3A) mutation associated with an unrelated mutation in the RPL3 gene.
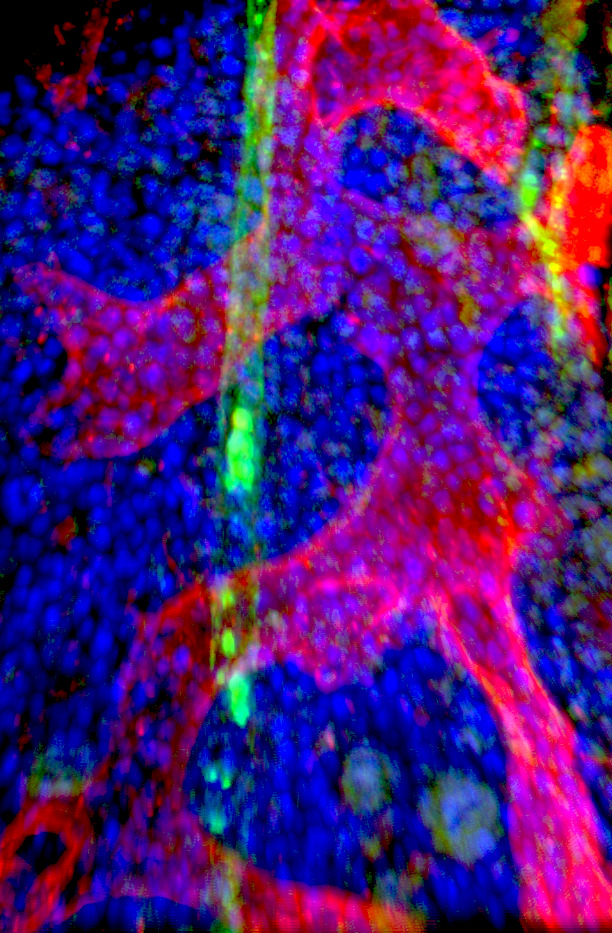
Next, they used single-cell transcriptome data to distinguish stem cells, progenitor cells, and leukemic blasts (white blood cells in bone marrow). Transcription factors, such as activator protein 1 (AP1), appear to be relevant in all, phenotypically very different, blast populations.
They integrated single-cell gene expression data and clonal tracking results to characterize cells as (pre-)leukemic stem cells or healthy cells. Importantly, MutaSeq data allowed for the comparison of gene expression between clones differing only in a single mutation. This enabled the researchers to determine the specific effects of a single mutation of hematopoiesis.
They found that mutations in the de novo DNA methyl transferase, DNMT3A, are the most common cause of benign clonal expansions of healthy stem cells in individuals of advanced age. Also, mutations in the multifunctional ribonucleoprotein 1 (NPM1) are identified as drivers for acute myeloid leukemia in 30% of patients and frequently co-occur with preleukemic DNMT3A mutations.
Lastly, the researchers compared gene expression between all (pre-)leukemic and nonleukemic cells, with the goal of identifying potential markers or drug targets present in all (pre-)leukemic cells, but not in residual healthy cells.
"There are a huge number of small molecule drugs out there with demonstrated clinical safety, but deciding which cancers and more specifically which patients these drugs are well suited for is a daunting task," explained author Lars Steinmetz, PhD, professor at Stanford University and group leader at EMBL Heidelberg, in a statement. "Our method can identify drug targets that might not have been tested in the right context. These tests will need to be carried out in controlled clinical studies, but knowing what to try is an important first step."
"We have now brought together clinical researchers from Germany and Spain to apply this method in much larger clinical studies," said author Lars Velten, group leader at the CRG. "We are also making the method much more streamlined. Our vision is to identify cancer stem cell specific drug targets in a personalized manner, making it ultimately as easy for patients and doctors to look for these treatments as it is testing for coronavirus".
Do you have a unique perspective on your research related to cancer research or genomics? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net