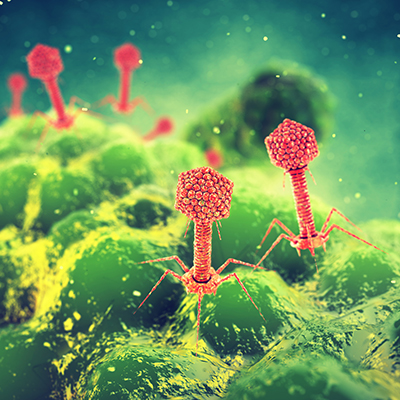
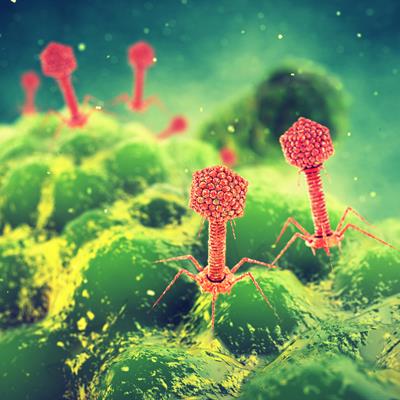
May 11, 2021 -- Adaptive Phage Therapeutics (APT) has closed $40.8 million in financing to accelerate the clinical development of its PhageBank phage therapies.
The company's PhageBank technology includes a collection of hundreds of bacteriophages that are collectively protective against six of the highest priority, multidrug-resistant bacterial pathogens. With the new funds, the company will advance clinical programs in prosthetic joint infection (PJI) and diabetic foot osteomyelitis (DFO), as well as for general corporate purposes, including further development of the proprietary PhageBank susceptibility test to rapidly identify phage therapies required to eradicate specific bacterial infections.
APT's technology was originally developed by the biodefense program of the U.S. Department of Defense. APT acquired the world-wide exclusive commercial rights in 2017.
The series B financing found was led by Deerfield Management.
Copyright © 2021 scienceboard.net