March 19, 2020 -- A long-standing hypothesis about the regulation of ion channels has been confirmed by cryoelectron microscopy (cryo-EM) imaging. The research presented in Nature on March 18 advances the basic understanding of key cellular processes that can be leveraged for a number of therapeutic and research applications.
Ion channels, transmembrane proteins with hydrophilic pores that facilitate transport of ions across cell membranes, are essential for a variety of cellular processes, including synaptic transmission and muscle contraction. In neurons, inactivation of both sodium and potassium channels is necessary for the generation of action potentials and regulation of synaptic firing.
Ion channels function by controlling the flow of ions in and out of a cell. But some ion channels, including those in neurons, require a means to quickly change the rate and amount of ion flow through channels to create currents caused by depolarization of cell membranes.
Researchers hypothesize that these channels often use a "ball-and-chain" mechanism to quickly inactivate channels to regulate the flow of ions. It has been proposed that in the inactivated state, which is stable and nonconducting, the pore is physically blocked by a "ball" of amino acids connected to a main protein by a string of residues on the cytoplasmic side of the membrane.
To confirm their hypothesis, researchers from Weill Cornell Medicine needed to directly image the mechanism on the atomic scale, which is notoriously difficult. The complexity of mammalian ion channels and the difficulty in reconstructing them for imaging purposes has made this task elusive for scientists in the past.
"Scientists have been trying to get an atomic-scale picture of this mechanism since the 1970s, and now that we have it at last, it can become an important drug target," said senior study author Crina Nimigean, PhD, an associate professor of physiology and biophysics in anesthesiology at Weill Cornell Medicine, in a statement.
Direct imaging of the ball-and-chain mechanism provides scientists with a new perspective on drug design. By targeting specific components of the ion channel, they may be able to improve their function, potentially enabling new therapies for a number of disorders and abnormalities, such as epilepsies, heart arrhythmias, schizophrenia, and diabetes.
To overcome imaging challenges, Nimigean and her team used cryoelectron microscopy to obtain structures of ion channels from Methanobacterium thermoautotrophicum, a bacteria-like species found at deep-sea geothermal vents. These purely calcium-gated and inactivating "MthK" channels are known to be structurally similar to mammalian "BK" potassium channels found in neurons and other cell types.
In contrast to MthK channels that are solely activated by calcium, BK channels are voltage-gated and can be activated by either calcium or electrical signals. The simplicity of MthK channels allows them to be investigated without the interference of other domains.
Using cryo-EM, the researchers were able to image key functional states in the calcium gating cycle of MthK channels, including the inactivated state. Via atomic and molecular simulations, the researchers were also able to show the channels in both an "open," or calcium-bound, state and a "closed" state in the absence of calcium.
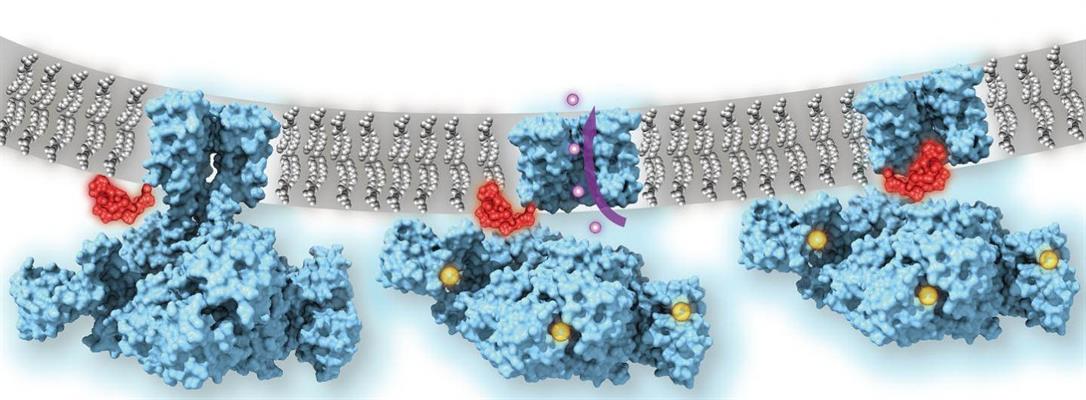
When calcium is bound to the regulator of potassium conductance (RCK) domains, the ring undergoes a large conformational change, or "gate rocking" that highlights the flexibility of the protein. The researchers classified three major subcategories of the "open" state based on the degree of tilt (approximately 20°, 8°, and 4° tilt, respectively).
The major conformation (approximately 80% of particles) observed showed that the TM2 helices (part of the channel pore) were wide open at the intracellular entryway. These changes were facilitated by the movement of the C-linker, which drew the RCK domains closer to the membrane and allowed the TM2 to kink open. The channel thus functions like a mechanical machine, translating calcium binding to protein unfolding and mechanical pulling on the gates to open the channel.
Molecular dynamics revealed that the "ball" protein, or the N terminus of TM1, is responsible for inactivation of the MthK channel as it binds in an extended conformation inside the pore. The researchers confirmed that the N terminus functioned as the plug in this mechanism by using stopped-flow fluorometry of wild-type MthK and constructs missing the 16 residues of the N terminus. While both channels activated into the open position, the MthK constructs missing the N terminus was not able to inactivate the channel, indicating that the N terminus peptide is responsible for inactivation of the MthK channel.
With this new information, Nimigean and her colleagues now are planning to explore how this mechanism might be targeted therapeutically.
"Different classes of potassium channels in human cells are very similar in their channel structures," Nimigean said. "So, a drug that blocks a particular channel will tend to affect other potassium channels and thus could have many unwanted side effects. However, understanding and then targeting this ball-and-chain structure that we were able to image could allow us to therapeutically modulate potassium channels with much more specificity."
Do you have a unique perspective on your research related to bioimaging or molecular biology? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net






