October 6, 2021 -- The world market for AI applications in drug development is projected to reach $5.1 billion by 2025, an increase of more than $3.6 billion from the market size in 2020. However, significant challenges are lurking ahead as the industry enters the next phase, according to Ulrik Kristensen, PhD, of Emersion Insights.
During the COVID-19 pandemic, there have been several positive developments for the AI drug development market. These include large funding rounds, an uptick in acquisitions and initial public offerings, and huge milestone deals that have matured and resulted in sizable payouts.
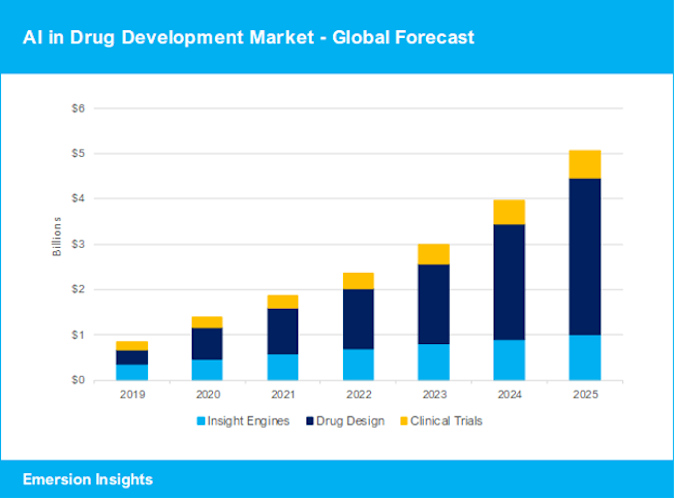
In terms of revenue growth, the AI drug design industry contributed massively to the increase in 2020. Milestones and royalties in the AI drug design industry gave clear indications of what we can expect in the future. While the overall AI drug development market grew 61.1% in 2020, AI drug designers enjoyed a staggering 130.1% growth.
Emersion Insights has categorized the AI drug development industry into insight engines, drug design, and clinical trials:
- Insight engines are AI platforms that aggregate and analyze information and real-world evidence from multiple sources, such as scientific literature, patient data, and clinical trial information, to find new associations and guide drug discovery or clinical trial optimization. Some of the insight engine vendors funded this year include Aetion, Cellarity, Causaly, and Elucidata.
- Drug design applications are using AI to develop new molecules, predict and optimize drug specificity and efficacy, or select drug repurposing options for existing drugs. Some of the drug design vendors funded this year include Insitro, Exscientia, and Insilico Medicine.
- Clinical trial applications help optimize the clinical trial process by improving patient stratification, with finer nuances to get the right patients for the right trials, to optimize enrollment and retention, and, in some cases, to help reduce the number of patients needed for a successful trial. Some of the clinical trial vendors funded this year include Medable, Mendel.ai, and Novadiscovery.
Looking ahead, AI drug design is expected to continue playing a major role in this market's growth. While insight engines and clinical trial solutions offer more predictability in income, successful AI drug designers have the potential to reach astonishing milestone payments and royalties. This has attracted a high number of risk-tolerant investors; 57% of the total investment in the industry has gone to AI drug designers. However, the potential reward comes with significant risk. With more than 130 AI drug designers to choose from, investors need to tread carefully in this industry.
Big pharma will continue to drive considerable growth in the years to come. While insight engines and clinical trial specialists will see continued revenue growth in the academic and healthcare segments as the need for higher efficiency and decentralization in clinical trials continues, milestone payments from big pharma to AI drug designers will remain a central contributor to the growth.
Challenges lurking ahead

Under the polished surface of revenue growth and massive funding, this industry is going to experience significant challenges in the coming years, including the following:
- A strong consolidation of the investments in the industry means a few front-runners now receive larger proportions of the overall funding, while late entrants struggle to keep up.
- The trend toward R&D outsourcing in big pharma has intensified in recent years, which means big pharma may be less keen on acquiring AI specialists than expected.
- For many of the pharmaceutical companies, prioritizing internal R&D capabilities, interoperability challenges, siloed data, and ongoing quantum computing readiness programs still need attention before they reach the full potential of AI acquisitions.
- After years of focusing on developing solutions and establishing initial partnerships, the competition among AI specialists has started to be reflected in revenue. Large milestone payments now differentiate the players and further amplify industry consolidation.
The largest and most successful AI firms are going for a long-term contract research organization (CRO) strategy, supporting the pharmaceutical industry as a separate entity. They have no intention of being acquired by their pharma partners. However, for many of the smaller players with strong solutions but less progression as far as business development, the best choice may often be to stay ahead of the inevitable consolidation and be acquired by these larger AI firms as they expand their capabilities.
As the industry enters the next phase, AI leaders are fine-tuning their strategic machinery. Many startups will fail, some will be acquired, and a few will grow to become highly profitable AI drug discovery CROs to the global pharmaceutical industry. And it's a prize worth the effort. These firms will tap into a $200 billion annual pharmaceutical R&D budget.
Ulrik Kristensen, PhD, is founder and principal analyst at Emersion Insights.
Do you have a unique perspective on your research related to artificial intelligence? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net







