October 22, 2020 -- ImmunityBio and NantKwest have launched phase I of a clinical trial for a COVID-19 vaccine candidate.
The first patient has received the second generation hAd5 COVID-19 vaccine, which targets both the outer spike protein and inner nucleocapsid of the SARS-CoV-2 virus. The two firms hope the vaccine will lead to long-term T cell and antibody immunity to the SARS-CoV-2 virus, the companies said.
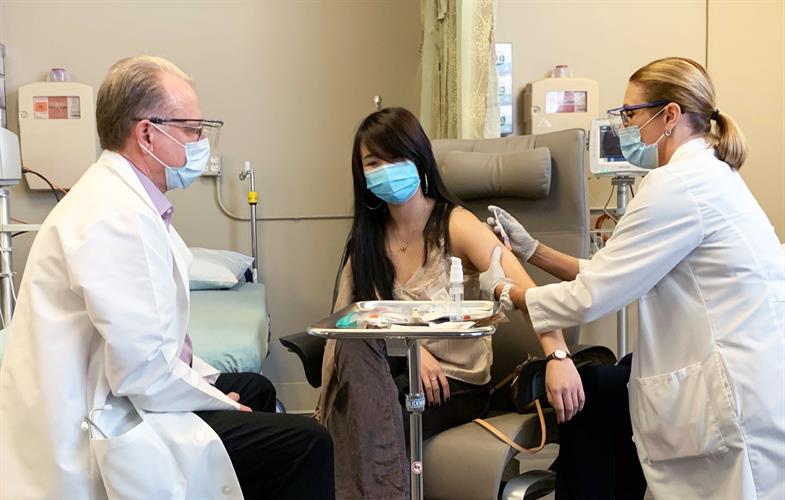
First patient receives California-based ImmunityBio's second-generation hAd5 COVID-19 vaccine, which targets both the outer spike protein and inner nucleocapsid, leading to potential long-term T cell and antibody immunity to the SARS-CoV-2 virus. Image courtesy of NantKWest.
The trial is being conducted at the Hoag Center for Research and Education in Newport Beach, CA, and is enrolling 35 healthy participants between the ages of 18 and 55.
Copyright © 2020 scienceboard.net
Member Rewards
Earn points for contributing to market research. Redeem your points for merchandise, travel, or even to help your favorite charity.
Research Topics
Interact with an engaged, global community of your peers who come together to discuss their work and opportunities.
Conferences
Connect
Tweets by @ScienceBoard