July 22, 2020 -- Conformational changes of the SARS-CoV-2 spike protein -- the target of many vaccines and therapies -- may have features that help the virus hide from the host immune system and survive longer in the environment, according to a study published in Science on July 21.
For enveloped viruses such as SARS-CoV-2, membrane fusion is a critical early step in viral entry to host cells. The fusion protein for SARS-CoV-2 is the spike protein, which mediates viral membrane fusion by refolding from a primed, semistable prefusion conformation to a stable postfusion state. The spike protein also induces neutralizing antibody responses in the host and is therefore an important target for vaccine development.
Binding of the spike protein through the receptor-binding domain of the S1 subunit to the host cell receptor (angiotensin-converting enzyme 2; ACE2) and subsequent proteolytic cleavage by serine proteases TMPRSS2 at a second site in the S2 subunit triggers dissociation and irreversible refolding into a postfusion conformation -- a trimeric hairpin structure. This change brings together viral and cellular membranes, ultimately leading to fusion.
Since the first genome sequences of SARS-CoV-2 were determined, several structures have been released, primarily of the prefusion stabilized conformation. The limited postfusion states have revealed that the spike protein undergoes a jack-knife transition. However, these structures have not provided enough resolution of viral membranes in the postfusion state to determine its structural and functional roles in viral entry.
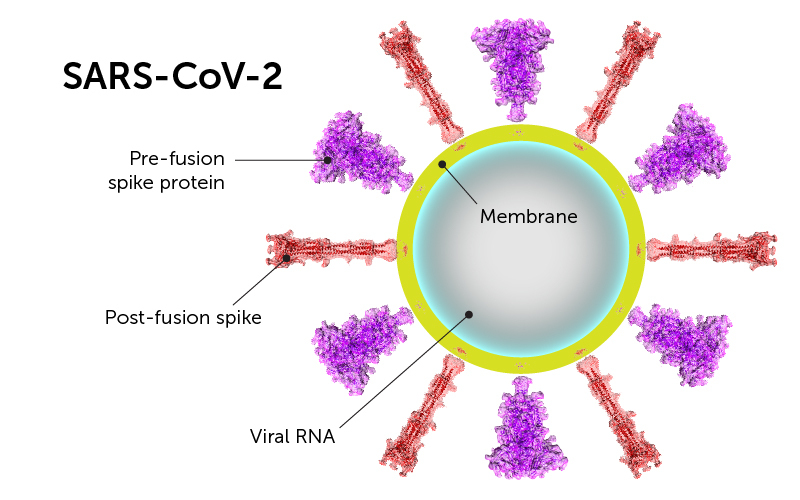
Therefore, researchers from Boston Children's Hospital determined the prefusion and postfusion states of the spike protein of SARS-CoV-2 using cryo-electron microscopy, and described their work in the Science paper.
Spontaneous shape-shifting as a defense mechanism
The researchers found that the spike protein can adopt the postfusion conformation in the absence of the cell receptor. This may occur by spontaneous dissociation of the S1 and S2 subunits, independent of target cells.
The interspersed rigid spike on the viral surface, from spontaneous postfusion conformations, may shield prefusion spike proteins from environmental conditions outside of the host. The authors suggested that the spontaneous shape change may prevent the SARS-CoV-2 virus from breaking down when it lands on a surface and may explain how the virus remains viable for several days outside of the host.
"Most viruses don't survive long outside the host," said lead author, Bing Chen, PhD, assistant professor of pediatrics at Harvard Medical School and scientist at Boston Children's Hospital, in a statement. "We think the rigid structure of these postfusion spikes protects the virus."
The postfusion conformation is very stable and is decorated with N-linked glycans (sugars). The researchers suggested that these glycans may play a protective role in inducing nonneutralizing antibody responses in order to evade the host immune system. When the postfusion conformation is prematurely formed, the postfusion spikes on the surface of mature and infectious SARS-CoV-2 virons may act as a decoy that distracts the immune system.
Implications for vaccine development
The research team believes that the spontaneous protective postfusion conformation of the spike protein may have serious effects on vaccine development programs. There are concerns that some vaccines may be developed using the postfusion form of the spike protein, which would lead to the formation of immunodominant, nonneutralizing epitopes that are not effective in blocking infection.
Vaccines that utilize a mix of prefusion and postfusion forms of the spike protein may be limited in their protective efficacy, the authors warned. Generally, they suggest that the number of postfusion S2 trimers in vaccine development should be minimized.
"We need to think about how to stabilize the spike protein," explained Chen. "If the protein is not stable, you may be able to induce antibodies, but they will be less effective in terms of blocking the virus. There may be batch-to-batch variation."
If the vaccines currently in development do not work well in phase III trials, the spontaneous shape-shifting of the SARS-CoV-2 spike protein may be the cause and the new mechanistic understanding may lead to better vaccine candidates, according to the authors.
Do you have a unique perspective on your research related to virology or molecular biology? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net