March 23, 2020 -- An international team of researchers has developed a unique library to the study regulation of the cytoskeletons of cells in space and time. The work, published in Nature Cell Biology on March 23, takes a systems-based approach to gain an overview of this process.
The cytoplasm provides a number of functions to cells. Primarily, it gives shape and mechanical resistance to deformation and plays an essential role in fundamental processes, including cell division and differentiation.
The Rho family of GTPases is a small family of guanine nucleotide-binding proteins that act as molecular switches to regulate organelle development, cytoskeleton dynamics, and cell movement. Defects in Rho signaling have been linked to cancer metastasis.
Their activity cycle is initiated by guanine nucleotide exchange factors (RhoGEFs) domains and terminated by GTPase-activating proteins (RhoGAPs) domains. These multidomain proteins contain 145 members that regulate Rho signaling. Until recently, these regulatory proteins have only been investigated individually, and only a few have been characterized.
"To understand complex processes, including cell shape changes, we need to know how the regulatory proteins work together collectively. Until now, we lacked a bird's eye perspective, so to speak," said senior study author Oliver Rocks, PhD, head of a Max Delbrück Center research group, in a statement.
In the current study, researchers from the Max Delbrück Center for Molecular Medicine and other institutions, such as the European Bioinformatics Institute (EMBL-EBI), sought to systematically characterize all the regulatory proteins to explore how signaling zones within a cell coordinate the cytoskeleton in space and time.
New models of Rho regulation
To characterize mammalian expression of RhoGEFs and RhoGAPs, the researchers generated an expression library of 141 full-length complementary DNAs. With this library they were able to see which molecular switches individual proteins control, where in cells this occurs, and which binding partners cells have.
The researchers then developed a screening-compatible live-cell imaging assay, using fluorescence resonance energy transfer (FRET)-based biosensors for the prototype GTPases RAC1, CDC42 and RHOA. Catalytic activities for 45 out of 75 RhoGEFs and 48 out of 63 RhoGAPs were found.
To understand how RhoGEFs and RhoGAPs contribute to spatial information to Rho signaling, the researchers mapped their subcellular distribution using confocal live-cell microscopy of yellow fluorescent protein (YFP) fusion proteins in epithelial cells. This analysis revealed that over half of RhoGEFs and RhoGAPs inherently localize to one or more distinct structures at steady state, thereby collectively present in virtually all cellular compartments.
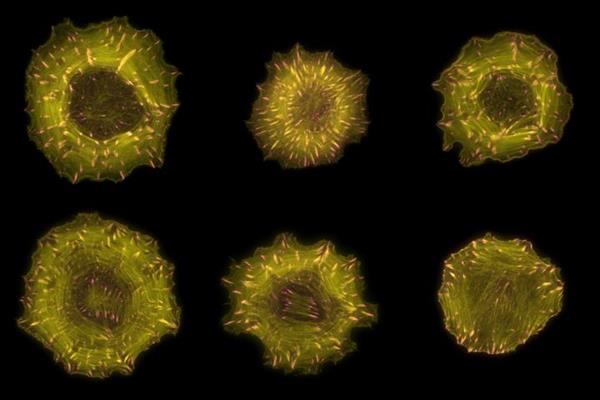
For example, researchers identified a total of 34 actin-associated proteins, only 12 of which were already known. Moreover, the interactomes of these 34 RhoGEFs and RhoGAPs were enriched in actin-binding proteins. These findings suggest that the close proximity of dedicated regulators to actin is important to locally sense and control cytoskeletal dynamics.
The researchers wanted to further explore potential stimulus-dependent or interaction-dependent relocalization of regulators. They investigated the consequences of receptor tyrosine kinase signaling and determined which regulators are recruited by the central adaptor protein GRB2 to the epidermal growth factor receptor.
Five out of the 25 potential GRB2-interacting regulators tested were directly associated with the adaptor and were corecruited to the plasma membrane following EGF stimulus. This suggests that most, if not all, Rho regulation occurs on dedicated cellular structures.
Focal adhesions dictate regulation
The cytoskeleton controls two opposing processes: cell protrusion and cell contraction. The researchers were interested in understanding how Rho GTPases coordinate these two spatially separated processes.
The spatial organization of the two opposing processes is made possible through focal adhesions, Rocks explained. Focal adhesions (FAs) are key sites of cytoskeletal dynamics and composed of protein accumulations near cell membranes.
During migration, FAs form near the front of a cell and mature into more stable structures as they move toward the middle of the cell, eventually dissolving. The researchers used total internal reflection fluorescence microscopy to determine that one-quarter of all regulators (37) associated with the FAs. This implies that FAs are central sites of Rho signaling regulation.
"We found a specific subgroup of regulators located almost exclusively on newly formed focal adhesions at the cell's edge and another separate subgroup on mature structures toward the middle of the cell," Rocks said.
These regulators directly translate the mechanical state of the cell into Rho activities that control protrusion-contraction balance in mechanosignaling. The researchers suggest that maturing focal adhesions serve as spatially organizing platforms that enable regulators to conduct mechanical cues and control cytoskeletal dynamics in migratory cells.
In the future, the researchers plan to investigate Rho GTPase regulations and their interactions with specific cytoskeletal machinery and how defective regulatory function can lead to disease.
"It was absolutely essential to revitalize this research field and open up new conceptual approaches for further study," Rocks said.
The database and protein library are now available to all scientists worldwide.
Do you have a unique perspective on your research related to bioinformatics or cell biology? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net



