April 24, 2020 -- What's the best strategy for developing treatments for the SARS-CoV-2 virus? The most promising approaches for a rapid response are antivirals and gene therapy, according to a new review article published April 24 in Frontiers in Microbiology.
In an unprecedented effort, hundreds of thousands of researchers and clinicians from around the world are fighting against the clock to develop cures, vaccines, and diagnostics for COVID-19, the illness caused by the virus SARS-CoV-2. To date, over 1,650 academic articles on COVID-19 have been published and accumulated in various databases. ClinicalTrials.gov lists over 460 ongoing clinical trials on several aspects of COVID-19, although most are still in the early stages.
Some of the most promising approaches are covered in the new article.
"To help focus the global search for a treatment, we here aim to provide a comprehensive resource of possible lines of attack against SARS-Cov-2 and related coronaviruses, including the results from all preclinical and clinical trials so far on vaccines against SARS and MERS," said senior author Ralph Baric, PhD, of the University of North Carolina at Chapel Hill, in a statement.
An elusive nature
A major challenge for the development of vaccines and antivirals against SARS-CoV-2 and other coronaviruses is the elusive nature of the viruses. They have high mutation and recombination rates that result in new viral strains that can rapidly adapt to new and changing ecologies. Therefore, they often emerge from highly heterogeneous populations of virus strains that circulate in animal reservoirs.
Vaccines and neutralizing antibodies that target the receptor-binding domain (RBD) of the spike (S) protein of coronaviruses can effectively neutralize the viruses. However, due to high selective pressure, the S protein is the most diverse region of coronaviruses.
For example, the S proteins of SARS-CoV and SARS-CoV-2 share 76% sequence identity, but the S proteins of SARS-CoV and MERS-CoV only share 44% sequence identity. This diversity of S proteins renders vaccines and neutralizing antibodies unlikely to be cross-protective between existing and emerging coronaviruses.
Current strategies for tackling viral outbreaks
In their review article, Baric and colleagues discussed one-by-one the possible strategies against the novel coronavirus, beginning with vaccine approaches. These can include inactivated vaccines, live attenuated vaccines, protein-based subunit vaccines (mimicking the RBD) with immune enhancers, viral-like particles, and nanoparticle vaccines.
Another approach is vector-based vaccines, the advantages of which include defined viral entry mechanisms and more efficient delivery of DNA into cells. Vectors can serve as adjuvants that elicit both B- and T-cell responses, and many vector systems have been used as vaccine platforms for other infectious diseases.
Many new vaccines specifically target SARS-CoV-2, including an RNA vaccine (mRNA-1273) currently in phase I clinical trials that encodes the prefusion-stabilized form of the SARS-CoV-2 S protein.
A second strategy while vaccines are in development is to use broad-spectrum antivirals to treat SARS-CoV-2. The high mutation rate of coronaviruses and the presence of quasispecies -- a population of viruses with a large number of variant genomes -- may interfere with the effectiveness of therapeutics.
The authors discussed how treatment with convalescent plasma derived from patients who have recovered from SARS-CoV and MERS-CoV that contains high titers of neutralizing antibodies can be a potent therapeutic, providing short-term passive immunity. Other antivirals that are being tested for efficacy against SARS-CoV-2 include fusion and viral protease inhibitors that block viral replication, host protease inhibitors, host receptor inhibitors, and immune modulators such as corticosteroids and interferons that boost host immune responses.
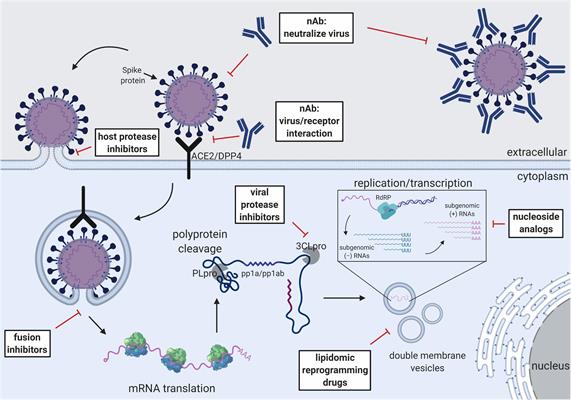
Broad-spectrum antivirals with efficacy against multiple coronaviruses could be a powerful tool in treating SARS-CoV-2. For instance, reports from several preclinical trials and a single clinical trial investigating SARS-CoV-2, SARS-CoV, and MERS-CoV indicate that remdesivir could be a promising treatment for SARS-CoV-2.
Gene therapies to address SARS-CoV-2
The researchers suggest that gene therapies could also hold potential in treating infectious diseases. Adeno-associated virus (AAV) vectors have been proven safe for human use, and multiple AAV-based gene therapy drugs are approved by the U.S. Food and Drug Administration and the European Medicines Agency. Given recent advancements in human antibody cloning technologies, AAV has promising potential to be a hybrid of vaccine and therapeutic that acts as a passive immunization vector to provide protection for an outbreak and act as a therapeutic in the early stages of disease.
Several AAV isolates are able to transduce human lung epithelial cells and evade humoral immune responses to allow for delivery of coronavirus vaccine or immunotherapeutic directly to the mucosal compartments of the lung. The package of neutralizing antibodies for delivery is extremely flexible, from authentic immunoglobulins to immunoadhesins to single-chain variable fragments to bispecific antibodies.
The authors suggest that AAV-based passive immunization could fill a gap left by prophylactic treatments by protecting the population before the arrival of a vaccine. Because AAV is a platform-based technology, anti-viral packages can be swapped and tested within a single month. The injection of neutralizing antibodies into lung tissue is not permanent, thereby reducing the chance that the host will produce antibodies against the AAV-based therapy.
"In theory, a single dose could mount a protective response within a week and last for more than a year. The currently high price could be reduced when treating infectious diseases, which have a larger market. It may or may not already be too late to use AAV to treat SARS-CoV-2, but it is certainly not too late for future outbreaks," said lead author Long Ping Victor Tse, PhD.
The passive immunization offered by AAV-therapies could be a quick and efficacious option to use during an outbreak situation for emerging diseases prior to vaccine deployment.
The researchers suggest that future vaccines and antivirals will need to be both potent and broadly effective against multiple potential emerging viruses within and across virus families. Treatments must also be readily available to affected populations and have fast response time.
Do you have a unique perspective on your research related to virology or drug development? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net


