December 13, 2021 -- A new method, which involves cooling living cells at extremely fast speeds, leads to unprecedented preservation of cellular biomolecules in their natural arrangement at the moment of arrest. Details of the technique were published in Science Advances on December 10.
A variety of techniques can be used to assess molecular patterns at micrometer and nanometer scales. Some of the techniques include fluorescence microscopy, single-molecule localization microscopy, nanoscopy with minimal photon flux, super-resolution radial fluctuation (SRRF), and stimulated emission depletion nanoscopy.
However, the spatial and spectroscopic resolutions of these imaging methods are limited by motional blur caused by finite photon fluxes and photobleaching (light-induced destruction). Because of these constraints, the transient states of many molecular patterns have remained largely inaccessible.
The problem with blur and photons in imaging
In fluorescence microscopy, exposure time can be prolonged to increase the amount of detected light. However, microscopic structures never stand still, but rather are constantly in motion. Prolonging the exposure time thereby leads to blurring of the structures.
Furthermore, the process of photon catalysis produces toxic radicals. This results in the destruction of molecular processes, the eventual death of cells, and even destruction of fluorescent molecules. Molecular motion can be superseded by chemical fixation, but the fixation process takes minutes and is incomplete, leading to inherent changes in the sample. On the other hand, cells can be cooled extremely fast, and yet mechanical destruction of cellular structures from ice crystals still occurs.
The solution may literally be very cool imaging
To overcome these fundamental limitations, researchers in the department of systemic cell biology at the Max Planck Institute of Molecular Physiology, led by Jan Huebinger, PhD, in the group of Philippe Bastiaens, PhD, have developed a technology called ultrarapid cryoarrest. Cryoarrest freezes molecular activity patterns within milliseconds directly on a fluorescence microscope.
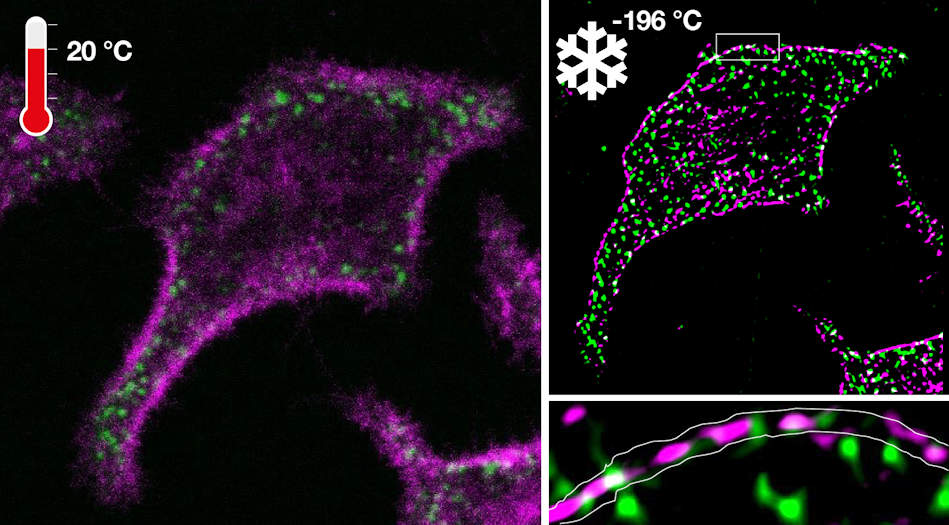
The researchers developed an ultrarapid cooling device that is integrated with a microscope, where the cooling effect of liquid nitrogen (-196° C) is accelerated under high pressure onto a diamond. This occurs at an extremely fast cooling rate of 200,000° C per second. The high-pressure burst, in combination with exceptional heat conductance of the diamond, enables the necessary cooling rates to arrest cells at -196° C in their native configuration.
Importantly, the ultrarapid cryoarrest technique allows scientists to analyze samples without the need for cryoprotectants. The technology can also be deployed on a multimodal fluorescence microscope that preserves the out-of-equilibrium molecular organization of living cells, which allows for the imaging of dynamic processes. Furthermore, increased spectroscopic resolution in fluorescence lifetime imaging microscopy made the acquisition of complete molecular reaction patterns possible.
Another unique characteristic of the system is that the same arrested cells could be observed by different microscopy modalities to measure patterns from the molecular to the cellular scale. This new technology thereby led to the discovery of nanoscopic co-organization of an oncoprotein (epidermal growth factor receptor, or EGFR) and a tumor suppressor protein (receptorlike protein tyrosine phosphatase γ, or R-PTP-γ) that safeguards cells from exhibiting malignant behavior.
The team observed patterns at multiple scales as demonstrated for the confocal laser scanning microscopy (CLSM)/SRRF (super-resolution radial fluctuations) and CLSM/STED (stimulated emission depletion) imaging of the EGFR/R-PTP-γ system.
"This is an enabling step for fluorescence microscopy, especially the combination of super-resolution microscopy and microspectroscopy that allow the mapping of molecular reactions in cells at multiple scales," Bastiaens said in a statement. "It will change the way we observe molecular organization and reaction patterns in cells and therefore provide more insight in the self-organizing capabilities of living matter."
Do you have a unique perspective on your research related to molecular biology and bioimaging? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net


