December 8, 2021 -- Researchers have bioengineered a metal-organic framework (MOF) antibody drug delivery system that has the potential to fast-track personalized therapies for cancer and other diseases. The findings were published December 7 in the journal Advanced Materials.
"The method offers the opportunity to personalize treatment and given the precision possible, may eventually change the current dosage needed for patients, resulting in fewer side effects and making treatments cheaper," said co-senior author Christoph Hagemeyer, PhD, in a statement. Hagemeyer is head of the NanoBiotechnology Laboratory at the Australian Centre for Blood Diseases at Monash University in Melbourne, Australia.
Antibodies as targeting agents
Antibodies are well known as therapeutic agents in their own right, but they can also be exploited as targeting agents that bind with high specificity to a wide range of target antigens.
This specificity makes antibodies an attractive targeting mechanism for precision medicine given that under conventional chemotherapy, just .01% of the drug currently reaches the targeted cancer tissue.
In previous research, antibodies have been applied to deliver a range of agents to disease sites, both for imaging or diagnostic purposes (e.g., delivery of fluorophores for fluorescence microscopy) and for therapeutic purposes (e.g., delivery of cytokines and pro-drug activation enzymes).
More recently, scientists have experimented with coupling antibodies with functional nanoparticles -- such as quantum dots, gold, or iron oxide nanoparticles -- for even higher specificity. However, for this to work, the antibody needs be positioned with a specific orientation, with the antigen-binding region protruding outward from the nanoparticle's surface.
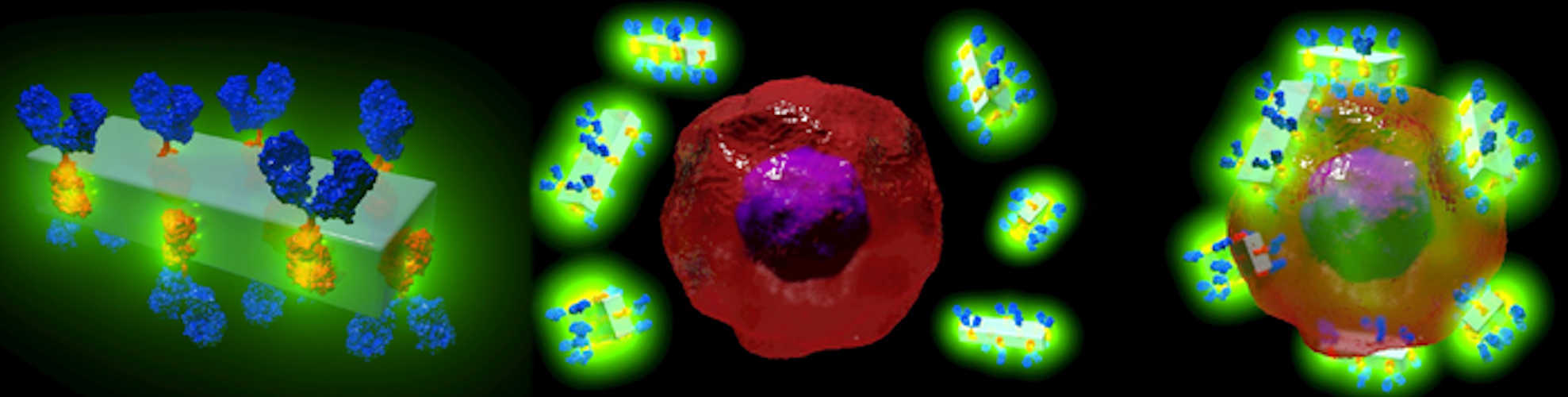
MOF nanocrystals decorated with antibodies
The paper describes how researchers took advantage of the electric charge properties of certain MOFs so that the antibodies attached to the MOF automatically oriented in the correct way. The research team developed the flat, plate-shaped MOF out of a mixture of metal (zinc) and carbonate ions, along with a small organic molecule (an imidazole, a colorless solid that is soluble in water).
For the paper, the scientists focused on the monoclonal antibody trastuzumab, which binds to the extracellular domain of the HER2 receptor (HER2-R), which is overexpressed in breast and other cancer types. They synthesized biocomposites (materials composed of a mix of natural and artificial constituents) consisting of the platelike MOF nanocrystals, along with the antibodies attached in the proper orientation (with the binding region protruding from the MOF surface toward the target).
Finally, to test the potential of the biocomposites for cellular delivery, the researchers performed an in vitro immunofluorescence assay that demonstrated that the biocomposite was efficiently internalized by only the targeted cancer (HER2-R+) cells (red in the figure). The in vitro study also showed that after the MOF antibody crystals bound to their target cancer cells, the low pH in the cells caused the crystal to break down.
"This simple synthetic approach has the potential to be extended from antibody-based sensing to diagnostic and therapeutic applications," the authors wrote.
Applications beyond cancer
Since monoclonal antibodies of all kinds are now ubiquitous and a cornerstone of biopharmaceuticals, the new MOF antibody drug delivery system should be applicable to a wide range of conditions outside of cancer, including chronic inflammatory, infectious, and cardiovascular diseases.
"With over 80 different monoclonal antibodies approved for clinical use, this approach has enormous potential to improve these antibodies for the targeted delivery of diagnostic agents and therapeutic drugs," said co-first author Karen Alt, PhD, in a statement. Alt is head of the Nano Theranostics Laboratory at the Australian Centre for Blood Diseases at Monash University.
"The goal is that ultimately the clinical translation of this technology will improve the quality of life for patients suffering from serious diseases," Alt said.
Do you have a unique perspective on your research related to drug discovery or personalized medicine? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net


