August 25, 2021 -- Researchers have grown 3D human brain tissue from stem cells that replicates the patterns of electrical activity found in the brains of patients with Rett syndrome. These "minibrain organoids" can be used to investigate the underlying causes of neurological disease and screen and test potential drug candidates, according to research published on August 23 in Nature Neuroscience.
Rett syndrome is a genetic disorder caused by de novo mutations in one copy of the MECP2 gene on the X chromosome. It mostly affects girls and is associated with learning delays, repetitive movements, and seizures characterized by distinctive bursts of chaotic electrical activity in the brain.
The brain of a Rett syndrome patient may appear normal on magnetic resonance imaging, with no overt abnormality in brain structure. This is why researchers need a reliable method to measure brain activity in the lab to understand and prevent seizures.
One of the best tools for studying the brain are "brain organoids," 3D brainlike structures grown from human stem cells. Until now, however, organoids were assumed to be unable to replicate brainwaves, the distinct patterns of neural oscillations associated with specific activities like sleeping, much less the coordinated waves of electrical activity that spread throughout the brain in response to stimuli, or in the case of Rett syndrome, the chaotic waves triggered by a seizure.
"With many neurological diseases, you can have terrible symptoms, but the brain physically looks fine," said first author Dr. Ranmal Samarasinghe, PhD, a member of the University of California, Los Angeles (UCLA) Broad Stem Cell Research Center, in a statement. "So, to be able to seek answers to questions about these diseases, it's very important that with organoids, we can model not just the structure of the brain but the function as well."
After producing a batch of brain organoids derived from the skin cells of healthy people, Samarasinghe and his colleagues used two different methods to study the patterns of electrical activity inside them. The first method, calcium sensor imaging, is a microscopy technique using fluorescent molecules that respond to the binding of Ca2+ ions. The second method, extracellular recordings of local field potentials, involves inserting a probe into the organoid to record brain activity and analyzing the resulting spectrograms.
The analysis demonstrated the presence of intricate network-level activities, including oscillatory rhythms resembling the patterns produced by electroencephalograms (EEGs).
"I hadn't anticipated the range of oscillation patterns we would see," said Bennett Novitch, PhD, a member of the UCLA Broad Stem Cell Research Center and senior author of the study.
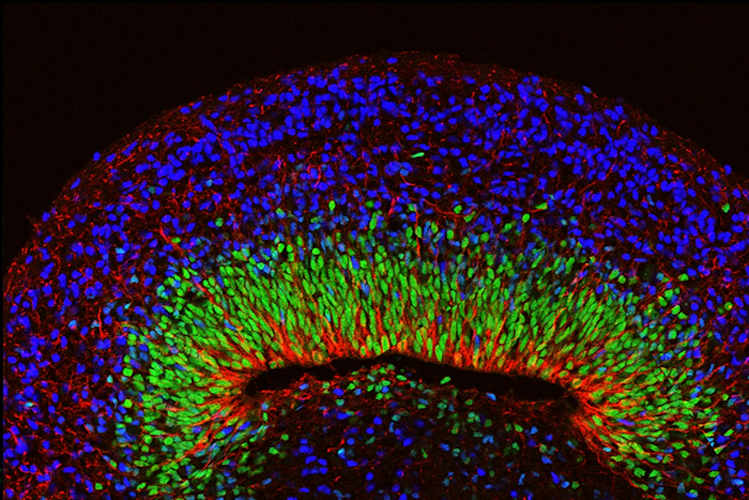
Next, the team developed brain organoids using cells from people with Rett syndrome. The organoids appeared outwardly normal, but when their neural oscillations were measured, the spectrograms showed spiky and chaotic ("epileptiform") electrical activity resembling the EEGs of people with Rett syndrome and related neurological disorders.
Finally, to test whether the organoids could be harnessed as a platform for drug testing, the researchers treated the Rett organoids with an experimental drug against Rett syndrome. The drug, pifithrin-alpha, is a target inhibitor of tumor protein p53 (TP53), which is the pathway through which the methyl-CpG binding protein 2 (MECP2) deficiency leads to overactivation in individuals with Rett syndrome.
Remarkably, application of the drug eliminated the seizure-associated activity patterns and caused the organoids' neural activity to become more normal.
"In summary, these findings illustrate the potential of brain organoids both as a unique platform for characterizing human neural networks and for personalized drug discovery and research," the authors concluded.
Do you have a unique perspective on your research related to drug development or neuroscience? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net