November 24, 2020 -- So-called "superspreader events" have been a major contributor to widespread transmission of the SARS-CoV-2 virus, according to a new analysis of outbreaks in Austria. Researchers used deep viral genome sequencing to trace the evolution of the pandemic in the country and how the virus spread beyond its borders, according to a study published in Science Translational Medicine on November 23.
Over the past months, scientists have determined that clustered outbreaks and superspreading events of SARS-CoV-2 infection pose a significant challenge to controlling the global COVID-19 pandemic. Despite the importance of these events, the scientific community still knows comparatively little about the fundamentals of the SARS-CoV-2 genome in the context of transmission dynamics within the human population.
Most of what is known about SARS-CoV-2 spread relates to acquired fixed mutations and phylogenetic analyses. However, low-frequency mutations and changes of the virus over time within individual patients can also provide insight into intra-host evolution, frequencies of viral populations in different groups, and fundamental differences in infection and pathogenicity of mutants.
Austria is centrally located in Europe with a population of 8.8 million. The country has a strong winter tourism season, which positioned it as a potential superspreading transmission hub in Europe in early 2020. During the first wave of the pandemic (February to May), winter tourism in Austria may have been responsible for up to half of the imported cases in Denmark, Norway, and other European countries.
Austria has a sophisticated healthcare system, including a national epidemiological surveillance program. As of August 7, 2020, contact tracing had been performed for all 21,821 reported SARS-CoV-2 positive cases. Out of these, 10,385 cases were linked to epidemiological clusters.
In the study, researchers from the CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences along with the Medical University of Vienna, the Austrian Agency for Health and Food Safety (AGES), and numerous universities and hospitals all over Austria, reconstructed major SARS-CoV-2 infection clusters in Austria to identify their role in international virus spread.
They utilized deep viral genome sequencing to analyze over 500 SARS-CoV-2 RNA samples from cases within the timeframe of the first wave. From the samples, the researchers assembled SARS-CoV-2 genome sequences, constructed phylogenies, and identified low-frequency mutations.
Retroactively observing the development of mutations
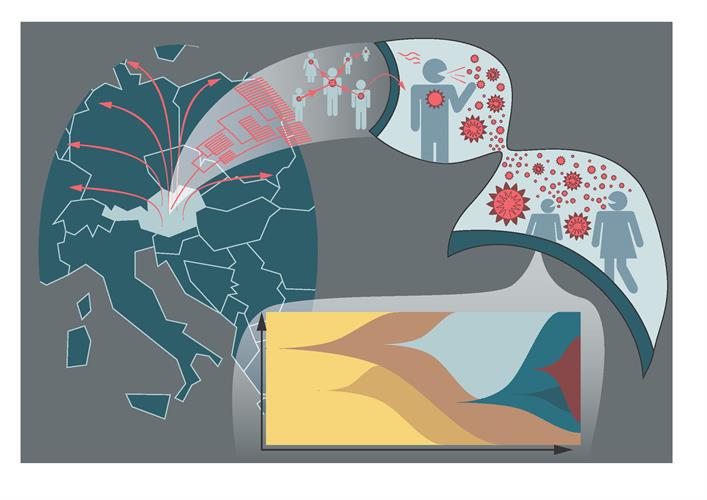
To investigate the link between local outbreaks and the global pandemic, the researchers performed phylogenetic analysis of 345 SARS-CoV-2 genomes from Austrian cases and 7,666 global genomes from the Global Initiative on Sharing All Influenza Data (GISAID) database. The team used mutation profiles along with geographical proximity of samples and time of infection as indicators for possible transmission links. From the samples, six clusters were linked to specific geographic locations, mainly the Tyrol region and Vienna.
The contact tracing program in Austria, along with phylogenetic analysis of the Austrian SARS-CoV-2 sequences, found strong overlap with 199 out of 345 sequences assigned to the two geographic clusters. The researchers suggested that the largest cluster in Tyrol originated from a single strain. This strain is now predominantly populated by strains from North America.
"This example illustrates how contact tracing and virus mutation analysis together provide a strong pillar of modern pandemic control," said lead author Andreas Bergthaler, PhD, principle investigator at CeMM, in a statement.
Comparing SARS-CoV-2 sequences in samples throughout Europe revealed that the emergence of the largest Tyrol cluster coincided with the local outbreak in France and with the early stages of the severe outbreak in northern Italy. There is more epidemiological evidence that the Tyrol outbreak in Austria then propagated to Iceland.
This same strain is based on the same mutation profile that could be found across continents. As a popular skiing destination, the town of Ischgl in the Tyrol region may have played a critical role as a transmission hub for the spread of clade 20C in Europe and beyond.
"Thanks to excellent epidemiological and our deep virus sequencing data, we could follow how the SARS-CoV-2 virus mutated in one individual and was then transmitted to others," Bergthaler explained.
On average 1,000 virus particles are transmitted during an infection
Lastly, the researchers performed time-resolved virus sequencing from 31 longitudinally sampled patients to reveal the viral mutation dynamics within individuals with COVID-19. Most patients showed a small number of stable low-frequency mutations with only a few cases exhibiting higher variability.
These differences may reflect the effect of host-intrinsic factors such as immune response or overall health, and extrinsic factors such as different treatment protocols, according to the authors. The low-frequency mutations become fixed in local clusters and then spread across countries, as was the case with the Tyrol cluster.
Furthermore, by analyzing epidemiologically validated infector-infectee pairs, the researchers determined an average of 1,000 SARS-CoV-2 particles are transmitted from one individual to another. This inter-human genetic bottleneck size for SARS-CoV-2 -- the number of virions that start infection and produce progeny in the viral population -- are much larger than other estimates for viruses, such as influenza A.
"Yet, occasionally we also found infected people who apparently came into contact with fewer virus particles and still became infected. We suspect that parameters such as the application of protective measures, the transmission route, or the immune system may play a decisive role here," Bergthaler added.
The study indicates that reducing the viral load of infected individuals by a combination of measures such as mask wearing, social distancing, and adequate indoor air exchange could play a key role in both preventing the spread of the virus and possibly even influence the course of the disease.
"The modern techniques of virus genome sequencing support epidemiological contact tracing and offer high-resolution insights of the ongoing pandemic," said co-author, Dr. Franz Allerberger, head of the public health division of AGES.
Do you have a unique perspective on your research related to virology or epidemiology? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net