March 31, 2020 -- A new therapeutic approach for suppressing seasonal influenza that involves synthetic phage shells that interfere with pathogen adhesion is immediately being tested for use on coronaviruses in response to the global COVID-19 pandemic. The research findings were published in Nature Nanotechnology on March 30.
Influenza viruses are responsible for over 650,000 deaths per year worldwide, according to the World Health Organization. While a number of antiviral therapies available, they are only partially effective, as lung cells are still significantly damaged.
With the COVID-19 pandemic claiming more than 33,000 lives (as of March 31) in over 200 countries, the need for viral prevention strategies is more urgent than ever before.
Targeted features of influenza viruses
In drug design, researchers seek to exploit the natural design of viruses. Both influenza viruses and coronaviruses contain class I fusion proteins, large transmembrane proteins on the viral envelope that facilitate viral entry.
The class I fusion protein of the influenza A virus, called hemagglutinin, has three binding sites that allow multivalent binding to terminal sialic acid residues of surface glycans of the host lung cell. Multivalent binding, or multiple simultaneous molecular interactions, allows tight binding between pathogens and host cells during the initial stages of infection.
It is this design that inspired German researchers to explore the possibility of developing an inhibitor that binds the trivalent receptors with a perfect fit, thereby perfectly simulating the surface of lung tissue cells. In this way, a phage capsid would be able to completely envelop a virus to prevent it from infecting human cells.
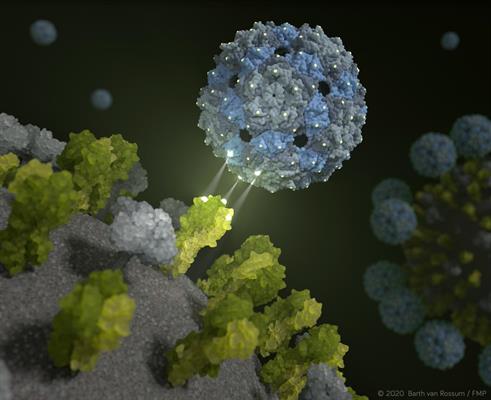
Chemical design of 'bait' capsids
The researchers identified the Q-beta bacteriophage as an ideal scaffold to present sialic acid ligands on their surface that match the binding sites of the trimeric hemagglutinin of the influenza A virus. The Q-beta capsid has a diameter of approximately 25 nm consisting of 180 copies of protein anchoring of sialic acid ligands with different linker lengths to match the distance between individual binding sites on the hemagglutinin trimer.
Next the researchers chemically modified the capsid using synthetic chemistry. Recombinant coat protein expression was achieved in Escherichia coli. Then sugar molecules (sialosides linked to ethylene glycol) were conjugated to the capsids through copper-catalyzed azide-alkyne cycloaddition.
The design of the new synthetic capsids was validated using high-resolution cryo-electron microscopy and cryo-electron microscopy. The researchers confirmed that the inhibitor completely encapsulates the virus.
Moreover, mathematical-physical models were used to simulate interactions between the influenza viruses and the phage capsid. This demonstrated that the rationally designed inhibitor does attach to hemagglutinin and completely envelopes the influenza virus.
Testing to see if the influenza virus takes the 'bait'
To prove that the structure of the designed phage was a potent inhibitor, the researchers conducted a number of in vitro, ex vivo, and in vivo experiments.
In vitro, infection with A/Panama/2007/1999 virus (A/Pan/99) and other avian influenza strains were inhibited by synthetic capsids in a dose-dependent manner. The synthetic capsids significantly reduced viral load in the supernatants of human lung tissues infected with the influenza A virus.
Of note, IFN-beta (interferon-beta) levels were significantly reduced after treatment with the capsid. This indicates that the antiviral activity of the phage capsids was conferred by binding to viruses and not mediated by antiviral cytokine induction or proinflammatory cell response.
Lastly, in vivo testing in mice showed that loss of body weight after infection was significantly reduced with pretreatment of the capsids.
The results of the cell culture studies revealed that the approach is biodegradable, nontoxic, and nonimmunogenic. The researchers believe that it can be applied to other viruses, and possibly bacteria. They indicate the next challenge they will test the approach on is the novel coronavirus, COVID-19, and they hope that the treatment could prevent coronaviruses from binding to host cells, thus preventing infection.
"Our rationally developed, three-dimensional, multivalent inhibitor points to a new direction in the development of structurally adaptable influenza virus binders. This is the first achievement of its kind in multivalency research," emphasized author Christian Hackenberger, PhD, head of the department chemical biology at the Leibniz-Forschungsinstitut für Molekulare Pharmakologie and Leibniz Humboldt Professor for Chemical Biology at Humboldt-Universität Berlin, in a statement.
Do you have a unique perspective on your research related to drug development or synthetic biology? Contact the editor today to learn more.
Copyright © 2020 scienceboard.net






