June 23, 2021 -- Chimeric antigen receptor (CAR) T-cell therapies have quickly become promising candidates for the treatment of many cancers. However, other immune cell types, such as natural killer (NK) cells, may be effective for some cancers, such as solid tumors, that are traditionally challenging for CAR T therapies. The science behind this concept is worth examining.
Traditional CAR T-cell therapy involves harvesting T cells from the patient and genetically modifying them to respond to specific cancer cell antigens. The cells are then expanded to large numbers before being reinfused into the patient. When these modified T cells encounter their target cancer cell antigen, they become activated, a process that directs them to proliferate and become cytotoxic, triggering a coordinated immune response against any cancer cells expressing the target antigen.
CAR T cells are modified to express cancer cell-specific CARs, which combine the functions of specific antigen binding and T-cell activation into a single receptor molecule. CARs are designed to mimic the T-cell receptors (TCRs) naturally found on T cells. TCRs are responsible for recognizing foreign antigens displayed on the surfaces of other cells, the first step of the T-cell activation process.
TCRs are composed of two polypeptide chains, each with constant (C) and variable (V) domains, with the variable domains of each TCR keyed to recognize a unique peptide sequence. To be recognized by a TCR, peptides must be processed and displayed by the major histocompatibility complex (MHC) molecules located on the surfaces of other cells. When the TCR recognizes its matching MHC-bound peptide, it interacts with other signal transduction molecules in the much larger TCR complex to initiate T-cell activation. CARs combine many of the functions of this large complex of proteins into a single polypeptide and are typically composed of the following four domains:
- The antigen binding domain is responsible for recognizing the target antigen and typically derived from an antibody binding site.
- The hinge domain is a short spacer sequence designed to provide mechanical flexibility to the receptor.
- The transmembrane domain is a hydrophobic region spanning the cell membrane, usually derived from the CD28 transmembrane domain.
- The intracellular domain is the intracellular signaling domain responsible for T-cell activation. (Early generations were derived only from the CD3-zeta cytoplasmic domain of the TCR complex, while later generations include other co-stimulatory domains such as CD28 or 4‐1BB.)
The resulting synthetic molecule behaves very much like a TCR complex, recognizing foreign antigens and initiating the T-cell activation process.
A CAR's antigen binding domain is typically a single-chain variable fragment (scFv) derived from an antibody. A human antibody consists of four polypeptide chains linked by disulfide bonds and two identical light and two identical heavy chains. Like TCRs, antibodies are made up of variable and constant domains. The light chains are composed of one variable domain and one constant domain (VL and CL), while heavy chains have one variable domain and three to four constant domains (VH, CH1, CH2, CH3). The antigen binding site is located within the variable domains (VL and VH), and a typical scFv combines the VL and VH domains into a single polypeptide with a short linker. Because the antigen binding site is derived from an antibody rather than a TCR, the antigen is not required to be presented by MHC to be recognized.
T cells may also be harvested from healthy donors (rather than the patient) in a method called allogeneic CAR T-cell therapy. A common side effect of allogeneic treatments is graft-versus-host disease, where the TCRs on the transplanted T cells recognize host-presented antigens as foreign and attack. This process can be curtailed by removing the TCR genes when the cells are modified to express CARs.
More recently, similar technology has been used to modify NK cells to express CARs. NK cells play a central role in the innate immune response, just as T cells play a key role in the adaptive immune response. NK-based therapies have several potential advantages over T cell-based therapies, including improved activity against solid tumors, reduced risk of cytokine storms, and lower neurotoxicity.
A collaboration to develop DAR-NK cells
San Diego-based Sorrento Therapeutics has recently entered into an agreement with NextGenNK Competence Center-associated research groups at the Karolinska Institutet (KI) Department of Medicine to develop novel cell-based therapeutics using NK cells derived from induced pluripotent stem cells.
In Sorrento's dimeric antigen receptor (DAR) design, one of the TCR chain genes is knocked out and replaced with a gene comprising the VH and CH1 domains. The VL and CL domains are expressed as a separate polypeptide which dimerizes with the VH and CH1 domains to form an antigen binding domain, just as they would in a normal antibody.
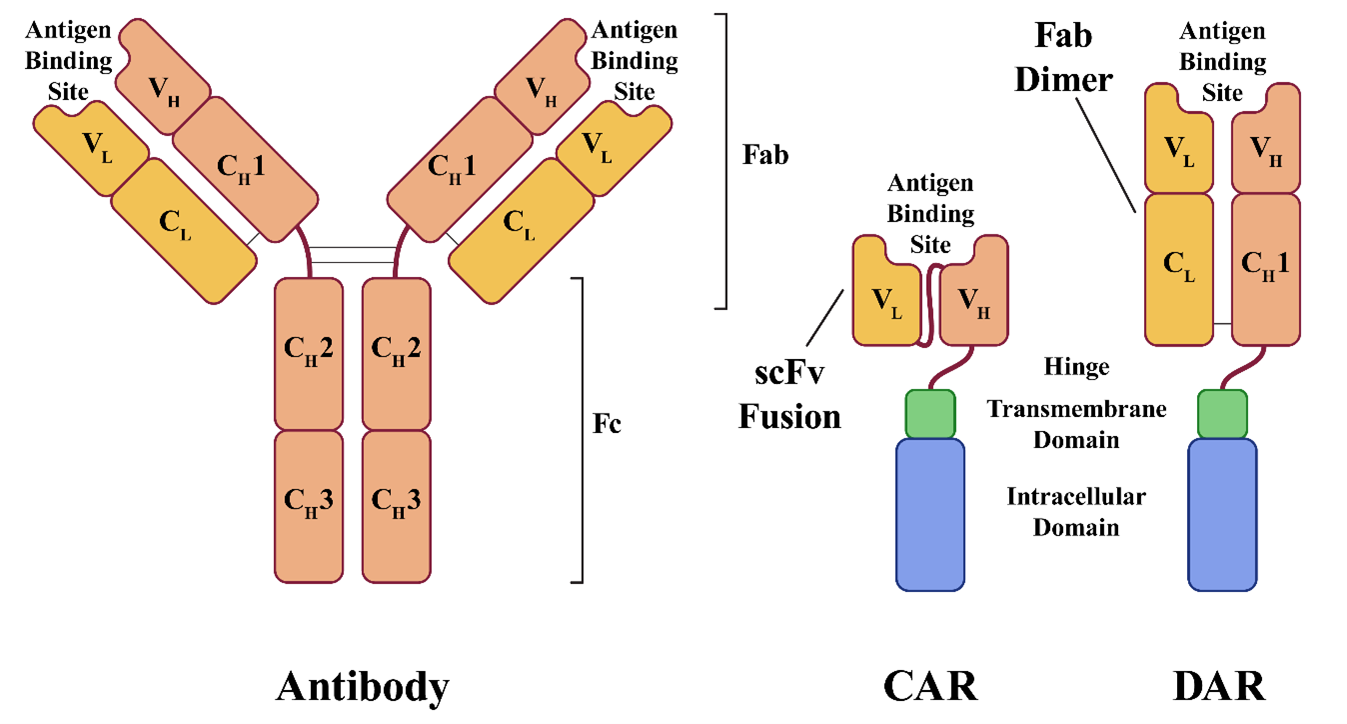
The result is an antigen binding domain built from a complete antigen-binding fragment (Fab), rather than the scFv fusion used in CAR design. Sorrento believes this design gives the binding site greater specificity, stability, and potency. The strategy also eliminates the original TCR, replacing it with the engineered DAR, making the technique useful for avoiding graft-versus-host disease in allogeneic treatments. Sorrento designed its DAR system with allogeneic therapies in mind and plans to use cells from healthy donors to create off-the-shelf treatments.
Sorrento and the KI plan to develop CAR-NK and DAR-NK therapeutics derived from induced pluripotent stem cells (iPSCs). iPSCs are somatic cells that have been reprogrammed to function as stem cells capable of differentiating into almost any cell type. Sorrento and KI intend to build off-the-shelf allogeneic therapeutics using NK cell lineages created from healthy donor cells through iPSC differentiation.
Blake Middleton is the editor of Cell and Gene Therapy Business Outlook, part of Science and Medicine Group.
Disclosure: Cell and Gene Therapy Business Outlook is a sister publication of ScienceBoard.net.
Do you have a unique perspective on your research related to cell therapy or immunotherapies? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net