September 18, 2019 -- A novel nanoparticle has been reported by researchers at San Diego State University with the potential to serve as a delivery system for therapeutics. The research published on September 17 in eLife describes how Metamorphosis Associated Contractile structures (MACs) trigger metamorphosis in tubeworms. Moreover, they identify a novel protein (with syringe-like structures, nicknamed Death Star for the effect it has) responsible for the activation of metamorphosis.
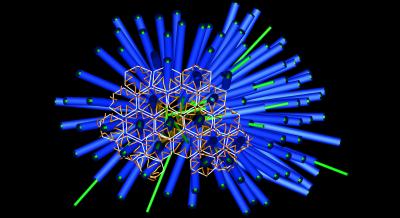
Tubeworms (Hydroides elegans) need a conducive environment to reproduce, so they typically gravitate to areas with healthy populations of bacteria like Pseudoalteromonas, a beneficial bacteria. MACs are found in many bacteria, including Pseudoalteromonas, and are examples of Contractile Injection Systems (CIS). These systems are macromolecular machines that puncture membranes to deliver proteinaceous effectors into target cells. They are often composed of an inner tube protein surrounded by a contractile, sheath, a tail-spike, and baseplate complex – forming a syringe-like structure.
In the current research, it was determined that MACs are responsible for injecting biochemicals into tubeworm larvae to help them transform into juvenile worms. This is an example of how CIS mediate trans-kingdom interactions bacteria and eukaryotes. Here, Pseudoalteromonas luteoviolacea (bacteria) contain a novel effector protein, Mif1. Metamorphosis is induced by delivering Mif1 to tubeworms (eukaryotes) via the tube lumen of MACs. "Lots of pathogens produce these syringe structures that typically cause disease," said Nicholas Shikuma, Assistant Professor at San Diego State University. "But this is the first time we discovered bacteria that use the syringe for a symbiotic purpose."
MACs resemble similar syringe structures found on bacteriophages and with evolution, bacteria have stolen this structure from the phages, and put it to good use. Researchers used cryo-electron tomography imaging, a technique where an electron microscope is used to record a series of two-dimensional images as a biological sample held at cryogenic temperatures is titled, and subsequent computational methods are used to yield a three-dimensional (tomographic) reconstruction of the sample. They found arrays of death star-shaped injection systems that are released by bacteria.
This research follows on another publications for the team in Cell Reports which investigated how extracellular CIS MACs target and inject an effector, Pne1, into mouse cell lines. This provided preliminary evidence for how payloads in MACs can be modified.
Scientists have identified these systems with the potential for re-engineering with medical and biotechnological applications, particularly being developed as delivery systems targeting eukaryotic cells. Extracellular CISs such as MACs are of particular interest, because they are released from the producing bacterial cell and autonomously bind to the target cell's surface. The research suggests that a number of effectors can be carried by MACs to provide a payload of choice. Not every bacteria in this strain produces the MACs, only about one out of 50 do so, but since trillions of these bacteria can be produced, supply will not be an issue and more of them can be engineered to produce MACs, explained Shikuma.
Shikuma has obtained a provisional patent for this system. Future research will focus on mining data from the Human Microbiome Project to see if humans have this same bacterial syringe structure in the GI tract that can be harnessed for therapeutics.
Do you have a unique perspective on your research related to drug discovery? The Science Advisory Board wants to highlight your research. Contact the editor today to learn more.
Copyright © 2019 scienceboard.net






