December 17, 2021 -- Cytovia Therapeutics and Cellectis have expanded their partnership on transcription activatorlike effector nuclease gene-edited NK cells derived from induced pluripotent stem cells to allow for a broader collaboration in China.
Under the amended agreement, Cellectis will receive $20 million in Cytovia stock, as well as up to $805 million in development, regulatory, and sales milestones, plus single-digit royalty payments. The agreement also adds a new CAR target, which will be developed in China by CytoLynx Therapeutics, Cytovia's joint venture.
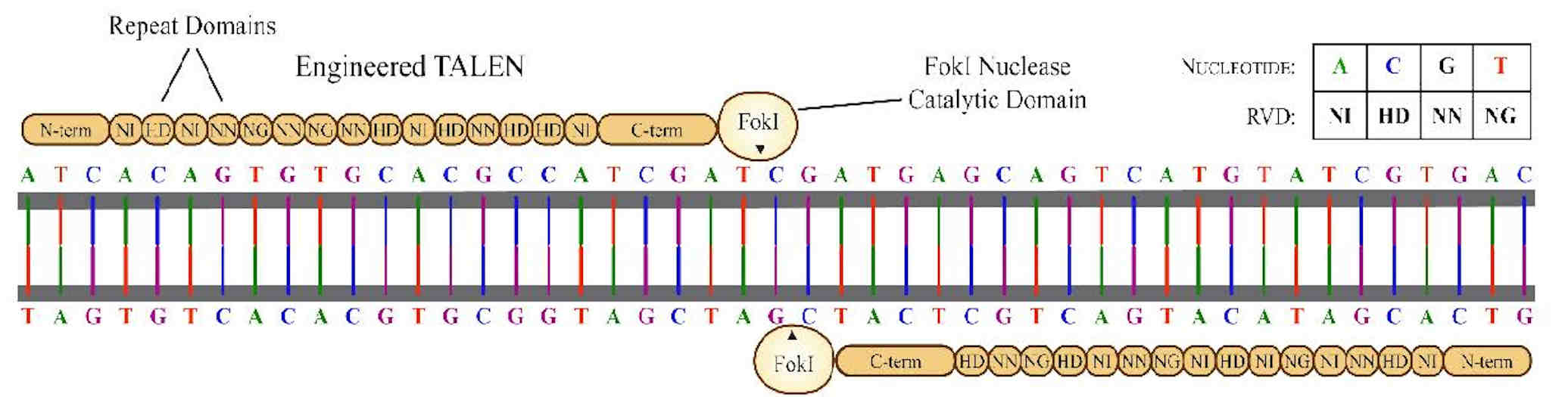
TALEN engineering is a custom nuclease technology that forms the basis of a powerful genome editing platform. Transcription activatorlike effectors (TALEs) are proteins found in Xanthomonas, a proteobacterium that infects plants.
TALEs are injected into plant cells and function as transcription activators of plant genes to facilitate infection. They contain a central domain that binds to a specific DNA sequence in the plant genome. That binding domain comprises several nearly identical repeats, each typically 33 or 34 amino acids long.
Each amino acid repeat is responsible for binding to a single DNA nucleotide, but only two variable amino acids within that repeat determine the nucleotide's specificity. Several different arrangements of these variable amino acids, which are called repeat variable diresidues (RVDs), have been discovered, but the four most abundant RVDs found in native TALEs are NI, HD, NN, and NG (binding to the nucleotides A, C, G, and T, respectively).
By altering the RVDs within each repeat, TALEs can be engineered to bind to almost any desired DNA sequence. These TALEs can then be fused to the nonspecific catalytic domain of a nuclease (such as the FokI restriction enzyme used in initial studies) to create a TALEN enzyme, a custom engineered endonuclease targeting almost any desired DNA sequence. When a TALEN is used in combination with a suitable DNA donor template, genetic insertions can be made via homology-directed repair.
Cellectis will develop custom TALENs, which Cytovia will use to edit iPSCs, which are somatic cells reprogrammed to function as stem cells capable of differentiating into almost any cell type. Cytovia is partnering with Justin Eyquem, PhD, at the University of California, San Francisco to apply precision gene editing to NK cells and identify optimal integration sites.
Cytovia's developmental pipeline is centered around two technology platforms.
iNK cells
NK cells play a central role in the innate immune response, just as T cells play a key role in the adaptive immune response. Upon activation, NK cells are capable of directly killing their target cells.
Cytovia is developing NK cells derived from iPSCs, and its developmental pipeline includes unedited iNK cells, TALEN gene-edited iNK cells with improved function and persistence, and TALEN gene-edited iNK cells (CAR-iNKs). CAR-NK cells are genetically modified to express cancer cell-specific CARs, which are artificially modified fusion proteins that combine the functions of specific antigen binding and NK cell activation into a single receptor molecule.
Cytovia plans to develop allogeneic, off-the-shelf, gene-edited iNK and CAR-iNK cells using Cellectis' TALEN gene-editing technology. Allogeneic CAR-NK cell therapy has the potential to target solid tumors with more efficient cytotoxicity while also offering lower neurotoxicity, a low risk of graft versus host disease, and a reduced risk of cytokine storms.
Flex-NK
Flex-NK engagers are multispecific trifunctional antibodies that function as NK cell engagers. The antibodies are chimeric fusions that work by binding to a specific cancer antigen in addition to two different NK cell-activating receptors. One region of the antibody binds to the specific cancer antigen, while another binds to NKp46, a natural cytotoxicity receptor on NK cells. An IL-15 moiety can also be cross-linked to the antibody to regulate activation and maintain homeostasis of the NK cells.
The FLEX platform was originally developed under the leadership of Cytovia's scientific co-founder Jean Kadouche, PhD, who is continuing to develop additional novel multifunctional formats for Cytovia. The NKp46 antibody is exclusively licensed from Yissum, a technology transfer company of the Hebrew University of Jerusalem. In April 2021, Yissum filed an international patent application for the NKp46 antibody.
Cytovia currently has several therapies in preclinical development based on these two platforms, including:
- CYT-303 (GPC3-Flex-NKp46), which is a tetravalent, bispecific, trifunctional antibody targeting Glypican-3 (GPC3), a glycoprotein highly expressed on solid tumors, including hepatocellular carcinoma (HCC).
- CYT-503 (GPC3 CAR iNK), a CAR NK therapy that targets GPC3 derived from TALEN-edited iPSCs for the treatment of HCC and other solid tumors.
- CYT-338 (CD38-Flex-NKp46), a tetravalent, bispecific, trifunctional antibody that targets CD38, a receptor highly expressed in multiple myeloma (MM).
- CYT-538 (CD38 CAR iNK), a CAR NK therapy that targets CD38 derived from TALEN-edited iPSCs for the treatment of MM.
- CYT-501 (EGFR vIII+wt CAR iNK), which is a CAR NK therapy targeting both the wild type (wt) and variant III (vIII) mutation of epidermal growth factor receptor (EGFR) derived from TALEN-edited iPSCs for the treatment of glioblastoma multiform and other solid tumors.
Cytovia is also developing two universally edited NK cell therapies, including:
- CYT-100, which uses universal iNK cells in combination with NK cell engagers for the treatment of solid tumors.
- CYT-150, which uses edited iNK cells in combination with NK cell engagers for the treatment of solid tumors.
For its part, Cellectis has several TALEN-edited CAR T-cell therapies in development in its universal chimeric antigen receptor T-cell (UCART) platform, which are off-the-shelf allogeneic product candidates. These candidates include the following:
- UCART123, which targets CD123, an antigen expressed on the surface of leukemic cells in acute myeloid leukemia (AML). The therapy is wholly owned by Cellectis and is currently in a phase I dose-escalation study in patients with relapsed/refractory AML. The first patient was dosed in January 2020.
- UCART22, which targets CD22, an antigen expressed on the surface of B cells from the pre-B cell stage of development through mature B cells. This occurrence happens in more than 90% of patients with B-cell acute lymphoblastic leukemia (B-ALL). The therapy is wholly owned by Cellectis and is now beginning a phase I, dose-escalation study in patients with relapsed/refractory B-ALL.
- UCARTCS1, which targets CS1/SLAMF7 (signaling lymphocytic activation molecule family member 7). CS1/SLAMF7 is an antigen that is overexpressed in MM. The therapy, owned by Cellectis, is currently in a phase I dose-escalation study in patients with relapsed/refractory MM. The first patient was dosed in October 2019.
- UCART19, which targets CD19, an antigen that is ubiquitously expressed during all phases of B-cell development. The therapy, initially developed by Cellectis, is now being jointly developed by Servier and Allogene. Allogene has exclusive rights in the U.S., while Servier retains exclusive rights for all other countries. UCART19 has completed two phase I studies in patients with relapsed/refractory B-ALL and is the most advanced allogeneic CAR T product in clinical development.
- ALLO-501, which also targets CD19 and features the same construct and editing process as UCART19 but with a different manufacturing process. The therapy is exclusively licensed from Cellectis and is being jointly developed by Servier and Allogene. Allogene has exclusive rights in the U.S., while Servier retains exclusive rights for all other countries. ALLO-501 is currently in a phase I study in patients with relapsed/refractory non-Hodgkin's lymphoma.
- ALLO-715, which targets B-cell maturation antigen (BCMA). BCMA is expressed on the surface of plasma cells, particularly on the surface of the malignant plasma cells that characterize MM. The therapy is exclusively licensed to Allogene and is currently in a phase I study in patients with relapsed/refractory MM.
In October 2021, the FDA placed a hold on all of Allogene Therapeutics' allogeneic CAR T-cell clinical trials following a report of a chromosomal abnormality in a patient treated with ALLO-501A, a modified offshoot of ALLO-501. Allogeneic CAR T-cells are treated as foreign cells and eventually cleared from the body by the host's immune system, so it is possible that the long-term health implications posed by this abnormality may be minimal.
Cellectis was founded in 1999 by André Choulika as a spinoff from the Pasteur Institute. Choulika was the first to use meganucleases to modify complex genomes and is considered to be the inventor of nuclease-based genome editing. When competition with CRISPR genome editing threatened Cellectis' prominence in the field, the company expanded its research into developing CAR T technologies for cancer immunotherapy.
Do you have a unique perspective on your research related to cell therapy? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net