August 20, 2021 -- Using tissue samples from cancer patients, Israeli researchers have successfully printed an active, viable glioblastoma 3D tumor model. In addition to potential applications in personalized medicine, the achievement promises to speed drug development and increase the chances that developed drugs will succeed in clinical trials. The results were published August 18 in Science Advances.
Current cancer models, especially traditional 2D petri dish tumor models, lack the tumor-stroma interactions that are essential for proper representation of the complexity of cancer biology. This inability to recreate the tumor microenvironment is one reason why drugs that show promise in laboratory research fail during clinical trials.
In this study, a team from Tel Aviv University recreated the dynamic tumor microenvironment by printing a 3D glioblastoma tumor on an EnvisionTEC 3D Bioplotter using two different bioinks: a tumor bioink comprising the natural polymers fibrinogen and gelatin and a sacrificial vascular bioink composed of a thermoreversible, biocompatible synthetic polymer.
To simulate a realistic tumor microenvironment, the team added patient-derived glioblastoma cells, astrocytes, and microglia to the 3D model. In addition, by coating the sacrificial vascular bioink with brain pericytes and endothelial cells, perfusable blood vessels were created through which blood cells and drugs could flow, adding further realism to the model.
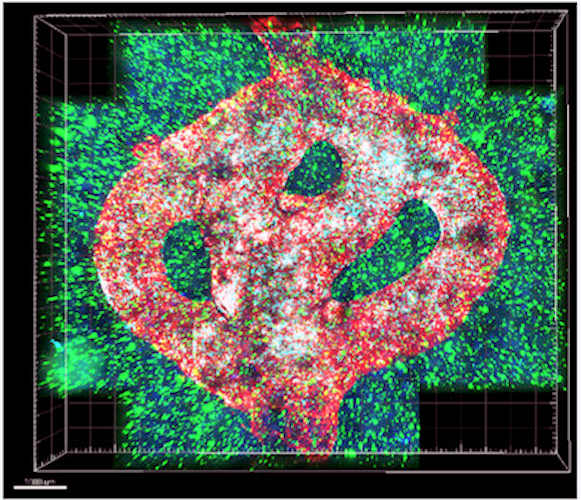
Glioblastoma is the most common, most aggressive, and deadliest form of malignant brain cancer. Scientists who study glioblastoma cells and develop treatments for it have been frustrated by the limitations of lab models developed in 2D plasticware models, which are unable to replicate the diverse effects of a given treatment in vivo.
"Cancer, like all tissues, behaves very differently on a plastic surface than it does in the human body," said lead researcher Ronit Satchi-Fainaro, PhD, head of the cancer research and nanomedicine laboratory and director of the Morris Kahn 3D Bioprinting for Cancer Research Initiative at Tel Aviv University, in a statement. "Approximately 90% of all experimental drugs fail at the clinical stage because the success achieved in the lab is not reproduced in patients. What happens is the cancer drugs will perform well in the plastic models, but that success does not translate over to the patients."
This problem is what inspired Satchi-Fainaro and her colleagues to develop a 3D bioprinted tumor model to use as a more realistic platform for rapid drug screening and for predicting the treatment outcomes of patients.
To test the clinical relevance of the 3D model, the researchers evaluated the response of patient-derived cancer cells to temozolomide (TMZ), a medication used to treat glioblastomas. Cells from three different patients were seeded into the 3D-printed tumor model and, for comparison, in a 2D plastic model (a 24 well plate). Then, different TMZ concentrations were applied for three days. To measure the efficacy of TMZ in each model, the researchers measured the corresponding half-maximal inhibitory concentrations (IC50 values).
The results showed that the IC50 values observed in 2D cultures were almost identical across the different patients' cells, whereas each cell type grown in the 3D-printed model exhibited a different IC50 value. This result is consistent with the observation that different patients can respond differently to the same therapy -- a phenomenon that forms the basis of personalized medicine. In fact, the three patients from whom the cells were derived all responded differently to their TMZ treatments, and each patient survived for a different period of time.
Satchi-Fainaro touted the potential applications of 3D-printed models to personalized therapy screening.
"If we take a sample from a patient's tissue, together with its extracellular matrix, we can 3D bioprint from this sample 100 tiny tumors and test many different drugs in various combinations to discover the optimal treatment for this specific tumor," she said.
The authors also envision 3D-printed models serving as platforms for drug discovery and development.
"We can test numerous compounds on a 3D bioprinted tumor and decide which is most promising for further development and investment as a potential drug," said Satchi-Fainaro.
Furthermore, in addition to replacing 2D plastic cultures, the authors suggested that 3D-printed models could replace animal models for preclinical cancer drug screening studies. In glioblastoma research, animal models are particularly difficult to establish and monitor, and many animals are required to achieve low-variance results, the authors wrote.
Do you have a unique perspective on your research related to drug discovery and development? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net







