February 24, 2021 -- Researchers have applied game theory in an effort to understand how SARS-CoV-2 mimics host proteins to support its own replication. The work, published in Royal Society Interface on February 24, applied a type of game theory on how information is signaled to reveal how the virus tries to trick human cells from attacking it.
"We need new models and technologies at many levels in order to understand how to tame viral pandemics," explained author Bud Mishra, PhD, a professor at New York University's Courant Institute of Mathematical Sciences, in a statement.
Mishra conducted the work in collaboration with William Casey, PhD, an assistant professor in the U.S. Naval Academy's Cyber Science Department, and Steven Massey, PhD, an assistant professor in the department of biology at the University of Puerto Rico.
The authors believed that signaling game theory can provide a framework for understanding deceptive signals that influence various situations. This theory involves manipulating the type of information transmitted between a sender and receiver to see how it can change the receiver's actions.
The authors suggested that information asymmetry -- incomplete information regarding a situation or object and differential states of knowledge held by participants -- occurs in nature and affects the fundamentals of the genome, biology, and even into human society.
A common form of deception is mimicry, where organisms imitate others in an attempt to increase their biological or evolutionary fitness. In their study, the researchers focused on two main types of mimicry: Batesian and Mullerian.
In Batesian mimicry, a "mimic" organism imitates a "model" organism in order to deceive a third organism. The outcome is a loss of fitness of the duped organism. An example of this is a harmless hoverfly that mimics a more dangerous wasp in order to deter predators.
In Mullerian mimicry, two organisms send a common signal to a third organism, which benefits all three. When two organisms share a common signal in a cooperative fashion, they may form a Mullerian ring. For instance, bees and wasps share black and yellow markings as a warning of toxicity to predators.
On a molecular basis, genes can be considered the sender/deceiver with the three-dimensional conformation of the expressed gene product acting as the signal, the authors explained. The receiver is a protein or other macromolecule that interacts with the modified product.
In their analysis, the authors constructed a mathematical model that mapped out a series of signaling strategies that, theoretically, could be adopted by both a virus (Batesian mimicry) and a vaccine (Mullerian mimicry).
Analyzing SARS-CoV-2 mimicry
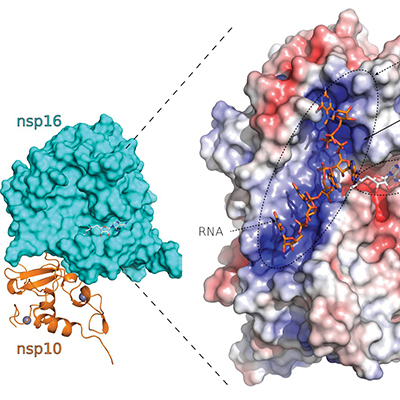
The SARS-CoV-2 virus uses multiple molecular mimicry tactics in its effort to exploit the human host. This includes the addition of a cap-like structure onto the 5' end of the viral messenger RNA (mRNA) by SARS-CoV-2 nsp16 that produces deceiver mRNAs to protect the virus from host innate immune responses. Additionally, the SARS-CoV-2 spike protein is glycosylated, which acts to further shield epitopes from recognition by the host immune system. These are examples of Batesian mimics.
Alternatively, polybasic cleavage sites (proteins that are cleaved by endogenous proteases) act as cooperative co-mimics, forming a molecular Mullerian mimicry ring. The SARS-CoV-2 spike protein also contains a polybasic cleavage site, which leads to the cleavage of the spike protein by the endogenous protein, furin, thus, increasing the infectivity of the virus. In a deceptive strategy, the spike polybasic cleavage site deceives furin in a Batesian fashion and invades the Mullerian ring.
The most potent weapon in the human arsenal against Batesian deception of the virus is molecular mimicry of the pathogen by vaccines. Vaccines can mimic different parts of the virus and after administration can deceive the human immune system into sensing it is being attacked by the virus. The costly short-term effect results in the immune system retaining memory so it is preprepared for a future encounter with the real virus.
However, the virus can circumvent vaccination through antigenic drift, in which the virus epitopes mutate over time, leading to novel immunogenic properties. According to the authors, the interplay between vaccines and virus demonstrates a co-evolutionary chase that oscillates between new vaccines and mutated virus.
The virus can also use decoy epitopes -- those deliberately exposed to the immune system, rather than being shielded by glycan -- to divert antibodies away from neutralizing epitopes. Identifying these decoy epitopes, may help researchers design more effective vaccines.
"Better knowledge of the deceptive strategies of SARS-CoV-2 will help to inform vaccine design," the researchers concluded.
Lastly, the authors noted that some antiviral drugs function as molecular mimics. For example, remdesivir is a molecular mimic of ribonucleotides. The drug represents a deceptive molecular signal, luring the virus replicase into binding to it. Yet, over time, the virus will develop resistance to the drug and eventually stop binding to the deceptive drug.
The virus may develop resistance to some drugs more slowly than others, the authors stated. For instance, if a drug follows polybasic cleavage site Mullerian mimicry ring, used by the spike protein, the signal sequence is constrained from diverging. This means that if the virus mutates this target sequence, it has negative effects on the host but also to itself.
The results of the mathematical analysis offer a range of blueprints of how mimicry is formed, maintained, and destroyed in cellular populations, with a special emphasis on pandemic viruses.
Do you have a unique perspective on your research related to virology or infectious diseases? Contact the editor today to learn more.
Copyright © 2021 scienceboard.net